Aspirin-mediated acetylation of haemoglobin increases in presence of high glucose concentration and decreases protein glycation
Q4 Biochemistry, Genetics and Molecular Biology
引用次数: 9
Abstract
Glycation represents the first stage in the development of diabetic complications. Aspirin was shown to prevent sugars reacting with proteins, but the exact mechanism of this interaction was not well defined. We performed a quantitative analysis to calculate the levels of acetylation and glycation of haemoglobin, among others red blood cell (RBC) proteins, using a label free approach. After glucose incubation, increases in the acetylation levels were seen for several haemoglobin subunits, while a parallel decrease of their glycation levels was observed after aspirin incubation. These results suggest that, a mutual influence between these two modifications, occur at protein level.
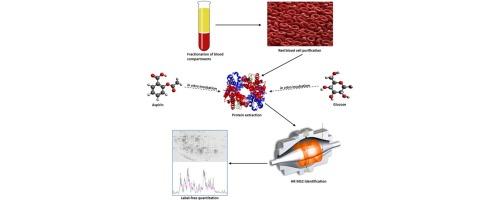
阿司匹林介导的血红蛋白乙酰化在存在高葡萄糖浓度时增加,并降低蛋白糖化
糖基化是糖尿病并发症发展的第一阶段。阿司匹林被证明可以防止糖与蛋白质发生反应,但这种相互作用的确切机制尚不清楚。我们进行了定量分析,以计算血红蛋白的乙酰化和糖化水平,以及其他红细胞(RBC)蛋白,使用无标记方法。葡萄糖孵育后,几个血红蛋白亚基的乙酰化水平升高,而阿司匹林孵育后,观察到它们的糖基化水平平行降低。这些结果表明,这两种修饰之间的相互影响,发生在蛋白质水平上。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

EuPA Open Proteomics
Biochemistry, Genetics and Molecular Biology-Biochemistry
自引率
0.00%
发文量
0
审稿时长
103 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


