The membrane complexome of a new Pseudomonas strain during growth on lysogeny broth medium and medium containing glucose or phenol
Q4 Biochemistry, Genetics and Molecular Biology
引用次数: 3
Abstract
In this study, we have performed a systematic analysis of Pseudomonas sp. strain phDV1 membrane protein complexes by growing the strain in lysogeny broth medium, and medium containing glucose or phenol as sole carbon sources. In order to study the membrane complexome, we developed an approach for the extraction and the analysis of the membrane protein complexes in native conditions. Our strategy involves (a) enrichment of the membrane proteome from Pseudomonas sp. strain phDV1 by two washing steps; (b) solubilization using n-dodecyl-β-maltoside; (c) a combination of BN-PAGE with Tricine-SDS-PAGE; and (d) protein identification of tryptic peptides by mass spectrometry.
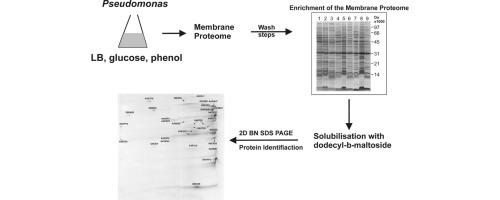
一株新假单胞菌在溶菌肉汤培养基和含葡萄糖或苯酚培养基上生长过程中的膜复合物
在本研究中,我们对假单胞菌菌株phDV1膜蛋白复合物进行了系统的分析,通过在溶原肉汤培养基和含葡萄糖或苯酚作为唯一碳源的培养基中培养菌株。为了研究膜复合物,我们开发了一种在天然条件下提取和分析膜蛋白复合物的方法。我们的策略包括:(a)通过两个洗涤步骤富集假单胞菌菌株phDV1的膜蛋白质组;(b) n-十二烷基-β-麦芽糖苷增溶;(c) BN-PAGE与Tricine-SDS-PAGE的组合;(d)质谱法鉴定色氨酸蛋白。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

EuPA Open Proteomics
Biochemistry, Genetics and Molecular Biology-Biochemistry
自引率
0.00%
发文量
0
审稿时长
103 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


