A simple method for cell culture of ‘Nemo’ ocellaris clownfish (Amphiprion ocellaris, Cuvier 1830)
Abstract
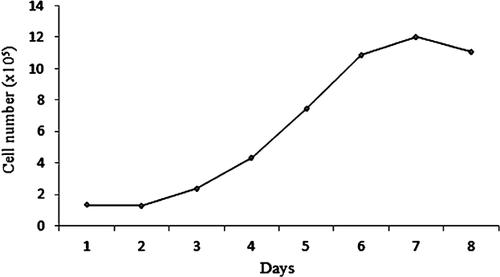
Worldwide, the ‘Nemo’ ocellaris clownfish (Amphiprion ocellaris, Cuvier 1830) is one of the top three most exported ornamental fishes. It also served as a subject in various fields of study except for cell culture. This first report described a simple explant method for culturing cells from the vertebra of ocellaris clownfish. The fish was first anesthetised with iced cold water and decapitated. The body trunk was disinfected in isopropanol and washed in sterile PBS. The vertebra was aseptically excised, washed two times in PBS and minced in the dissection solution (PBS containing 250 IU/mL penicillin, 250 µg/mL streptomycin, 50 µg/mL gentamycin and 2.5 µg/mL amphotericin-B). Then, the vertebral biopsies were washed three more times in PBS before being seeded in 25 cm2 culture flasks containing 1.5 mL of RPMI-1640 supplemented with 20% FBS. A small amount of CO2 was injected into the flask before it was tightly capped and incubated at 28°C in the regular incubator. When the monolayer reached 40–50% confluence, the vertebral biopsies were dislodged together with the medium to initiate a new primary culture. The cell monolayer was subcultured with short, cold 0.05% trypsin. The Nemo cell line was grown in the medium containing 15% FBS. The cell line at passage 4 had the population doubling time of 39.6 h and the cell line at passage 5 could be cryopreserved with 80% viability. This simple and reliable explant method has been applied successfully to culture cells of both marine and freshwater fishes for the prometaphase chromosome preparation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


