Unusual Transformations of Mono- and Disaccharide Intermediates in the Synthesis of Oligosaccharides Related to Fragments of the Capsular Polysaccharide of Haemophilus influenzae Type e
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
In the course of synthesis of oligosaccharides related to fragments of the capsular polysaccharide of Haemophilus influenzae type e, reactions of 2-O-trifluoromethanesulfonate β-D-glucopyranoside derivatives with the azide anion were studied. The reactions gave products of both nucleophilic substitution and rearrangement accompanied by 6-membered pyranose ring contraction to a 5-membered ring through (O5–C2)-cyclization. The formation of these products was interpreted for the first time using quantum mechanical calculations.
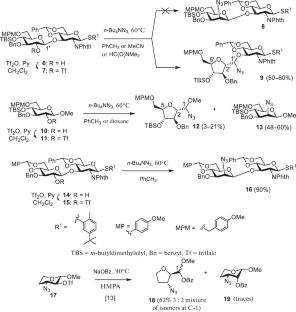
与流感嗜血杆菌荚膜多糖片段相关的低聚糖合成中单糖和双糖中间体的异常转化
在流感嗜血杆菌e型荚膜多糖片段相关寡糖的合成过程中,研究了2- o -三氟甲磺酸β- d -葡萄糖苷衍生物与叠氮阴离子的反应。该反应产生亲核取代和重排产物,并伴随6元吡喃环通过(O5-C2)环化收缩成5元环。这些产物的形成第一次用量子力学计算来解释。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


