Baljinder K Bains, Michelle K Greene, Leona M McGirr, Jay Dorman, Stuart N Farrow, Christopher J Scott
{"title":"Encapsulation of the p38 MAPK inhibitor GSK 678361A in nanoparticles for inflammatory-based disease states","authors":"Baljinder K Bains, Michelle K Greene, Leona M McGirr, Jay Dorman, Stuart N Farrow, Christopher J Scott","doi":"10.1002/jin2.9","DOIUrl":null,"url":null,"abstract":"<div>\n \n <p>Inhibitors of p38 mitogen-activated protein kinase (MAPK) are currently being pursued as therapeutics in inflammatory conditions, but many candidates have demonstrated limited efficacy or toxicity issues to date. Nanoformulation of p38 MAPK inhibitors may overcome these challenges, by enabling controlled release and targeted delivery. Thus, the aim of this study was to develop a nanoformulation of the p38 MAPK inhibitor GSK 678361A and subsequently validate its anti-inflammatory efficacy in vitro, versus the drug in its free format. Poly(lactic-co-glycolic acid) nanoparticles encapsulating GSK 678361A were prepared via a salting-out method and characterised by photon correlation spectroscopy, scanning electron microscopy and high-performance liquid chromatography. The anti-inflammatory effect of both free and nanoformulated GSK 678361A was evaluated in cultures of lipopolysaccharide-stimulated macrophages, with subsequent enzyme-linked immunosorbent assay analysis of TNF-α and IL-6 providing readouts of efficacy. A controlled release nanoformulation of GSK 678361A was successfully developed, with physicochemical characterisation revealing an average particle diameter of 115.5 ± 3.5 nm and polydispersity index of 0.13 ± 0.03, indicative of a homogeneous size distribution. GSK 678361A loading was quantified at 10.1 ± 0.4 µg per mg of poly(lactic-co-glycolic acid), equating to an entrapment efficiency of approximately 50%. When tested in cultures of lipopolysaccharide-stimulated macrophages, GSK 678361A nanoparticles inhibited the production of pro-inflammatory cytokines to an extent that was largely comparable with the free drug, although superior efficacy of the nanoformulation was observed at selected doses. These studies indicate that GSK 678361A may be successfully nanoformulated without loss of drug activity, warranting further evaluation in models of inflammation in vivo. © 2016 The Authors. Journal of Interdisciplinary Nanomedicine published by John Wiley & Sons Ltd and the British Society for Nanomedicine</p>\n </div>","PeriodicalId":91547,"journal":{"name":"Journal of interdisciplinary nanomedicine","volume":"1 3","pages":"85-92"},"PeriodicalIF":0.0000,"publicationDate":"2016-05-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://sci-hub-pdf.com/10.1002/jin2.9","citationCount":"8","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Journal of interdisciplinary nanomedicine","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jin2.9","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 8
Abstract
Inhibitors of p38 mitogen-activated protein kinase (MAPK) are currently being pursued as therapeutics in inflammatory conditions, but many candidates have demonstrated limited efficacy or toxicity issues to date. Nanoformulation of p38 MAPK inhibitors may overcome these challenges, by enabling controlled release and targeted delivery. Thus, the aim of this study was to develop a nanoformulation of the p38 MAPK inhibitor GSK 678361A and subsequently validate its anti-inflammatory efficacy in vitro, versus the drug in its free format. Poly(lactic-co-glycolic acid) nanoparticles encapsulating GSK 678361A were prepared via a salting-out method and characterised by photon correlation spectroscopy, scanning electron microscopy and high-performance liquid chromatography. The anti-inflammatory effect of both free and nanoformulated GSK 678361A was evaluated in cultures of lipopolysaccharide-stimulated macrophages, with subsequent enzyme-linked immunosorbent assay analysis of TNF-α and IL-6 providing readouts of efficacy. A controlled release nanoformulation of GSK 678361A was successfully developed, with physicochemical characterisation revealing an average particle diameter of 115.5 ± 3.5 nm and polydispersity index of 0.13 ± 0.03, indicative of a homogeneous size distribution. GSK 678361A loading was quantified at 10.1 ± 0.4 µg per mg of poly(lactic-co-glycolic acid), equating to an entrapment efficiency of approximately 50%. When tested in cultures of lipopolysaccharide-stimulated macrophages, GSK 678361A nanoparticles inhibited the production of pro-inflammatory cytokines to an extent that was largely comparable with the free drug, although superior efficacy of the nanoformulation was observed at selected doses. These studies indicate that GSK 678361A may be successfully nanoformulated without loss of drug activity, warranting further evaluation in models of inflammation in vivo. © 2016 The Authors. Journal of Interdisciplinary Nanomedicine published by John Wiley & Sons Ltd and the British Society for Nanomedicine
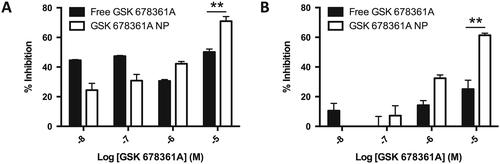
p38 MAPK抑制剂GSK 678361A在炎症性疾病状态的纳米颗粒包封
p38丝裂原活化蛋白激酶(MAPK)抑制剂目前正被用作治疗炎症的药物,但迄今为止许多候选药物的疗效有限或存在毒性问题。p38 MAPK抑制剂的纳米制剂可以通过控制释放和靶向递送来克服这些挑战。因此,本研究的目的是开发p38 MAPK抑制剂GSK 678361A的纳米配方,并随后在体外验证其抗炎功效,与自由形式的药物相比。采用盐析法制备了包封GSK 678361A的聚乳酸-羟基乙酸纳米颗粒,并用光子相关光谱、扫描电镜和高效液相色谱对其进行了表征。在脂多糖刺激的巨噬细胞培养中,对游离和纳米配方GSK 678361A的抗炎作用进行了评估,随后对TNF-α和IL-6进行酶联免疫吸附分析,得出疗效。成功制备了GSK 678361A控释纳米制剂,理化表征结果表明,该制剂的平均粒径为115.5±3.5 nm,多分散性指数为0.13±0.03,粒径分布均匀。GSK 678361A在10.1±0.4µg / mg聚(乳酸-共乙醇酸)下被定量,相当于约50%的包封效率。当在脂多糖刺激的巨噬细胞培养物中进行测试时,GSK 678361A纳米颗粒抑制促炎细胞因子的产生的程度与游离药物相当,尽管在选定剂量下观察到纳米制剂的优越疗效。这些研究表明,GSK 678361A可能在不丧失药物活性的情况下成功制成纳米配方,值得在体内炎症模型中进一步评估。©2016作者。跨学科纳米医学杂志,John Wiley &Sons有限公司和英国纳米医学协会
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


