Microbial drivers of DMSO reduction and DMS-dependent methanogenesis in saltmarsh sediments
IF 10.8
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 0
Abstract
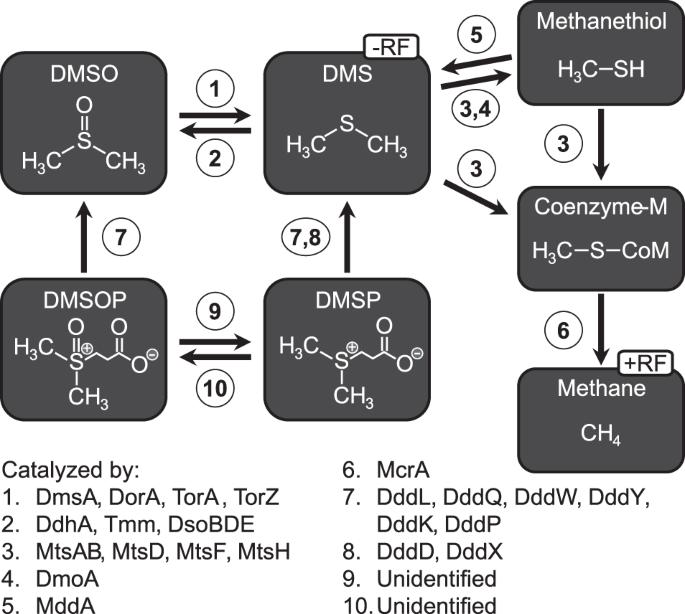
Saltmarshes are highly productive environments, exhibiting high abundances of organosulfur compounds. Dimethylsulfoniopropionate (DMSP) is produced in large quantities by algae, plants, and bacteria and is a potential precursor for dimethylsulfoxide (DMSO) and dimethylsulfide (DMS). DMSO serves as electron acceptor for anaerobic respiration leading to DMS formation, which is either emitted or can be degraded by methylotrophic prokaryotes. Major products of these reactions are trace gases with positive (CO2, CH4) or negative (DMS) radiative forcing with contrasting effects on the global climate. Here, we investigated organic sulfur cycling in saltmarsh sediments and followed DMSO reduction in anoxic batch experiments. Compared to previous measurements from marine waters, DMSO concentrations in the saltmarsh sediments were up to ~300 fold higher. In batch experiments, DMSO was reduced to DMS and subsequently consumed with concomitant CH4 production. Changes in prokaryotic communities and DMSO reductase gene counts indicated a dominance of organisms containing the Dms-type DMSO reductases (e.g., Desulfobulbales, Enterobacterales). In contrast, when sulfate reduction was inhibited by molybdate, Tor-type DMSO reductases (e.g., Rhodobacterales) increased. Vibrionales increased in relative abundance in both treatments, and metagenome assembled genomes (MAGs) affiliated to Vibrio had all genes encoding the subunits of DMSO reductases. Molar conversion ratios of <1.3 CH4 per added DMSO were accompanied by a predominance of the methylotrophic methanogens Methanosarcinales. Enrichment of mtsDH genes, encoding for DMS methyl transferases in metagenomes of batch incubations indicate their role in DMS-dependent methanogenesis. MAGs affiliated to Methanolobus carried the complete set of genes encoding for the enzymes in methylotrophic methanogenesis.
盐沼沉积物中DMSO还原和DMS依赖性甲烷生成的微生物驱动因素。
盐沼是高产环境,表现出高丰度的有机硫化合物。二甲基亚磺基丙酸酯(DMSP)由藻类、植物和细菌大量生产,是二甲基亚砜(DMSO)和二甲基硫化物(DMS)的潜在前体。DMSO作为厌氧呼吸的电子受体,导致DMS的形成,DMS被甲基营养原核生物释放或降解。这些反应的主要产物是具有正(CO2、CH4)或负(DMS)辐射强迫的微量气体,对全球气候具有相反的影响。在这里,我们研究了盐沼沉积物中的有机硫循环,并在缺氧分批实验中跟踪了二甲基亚砜的还原。与之前从海水中进行的测量相比,盐沼沉积物中的二甲基亚砜浓度高出约300倍。在分批实验中,DMSO被还原为DMS,随后伴随CH4的产生而被消耗。原核生物群落和二甲基亚砜还原酶基因计数的变化表明,含有Dms型二甲基亚硫还原酶的生物体(如脱硫菌、肠杆菌)占主导地位。相反,当钼酸盐抑制硫酸盐还原时,Tor型DMSO还原酶(例如红细菌门)增加。在两种处理中,弧菌的相对丰度都有所增加,属于弧菌的宏基因组组装基因组(MAG)具有编码DMSO还原酶亚基的所有基因。摩尔转化率为4/添加的DMSO伴随着甲基营养产甲烷菌Methanosarcinales的优势。批培养的宏基因组中编码DMS甲基转移酶的mtsDH基因的富集表明它们在DMS依赖性甲烷生成中的作用。隶属于甲烷菌的MAG携带一整套编码甲基营养甲烷生成酶的基因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ISME Journal
环境科学-生态学
CiteScore
22.10
自引率
2.70%
发文量
171
审稿时长
2.6 months
期刊介绍:
The ISME Journal covers the diverse and integrated areas of microbial ecology. We encourage contributions that represent major advances for the study of microbial ecosystems, communities, and interactions of microorganisms in the environment. Articles in The ISME Journal describe pioneering discoveries of wide appeal that enhance our understanding of functional and mechanistic relationships among microorganisms, their communities, and their habitats.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


