Remediation of acid mine drainage and immobilization of rare earth elements: Comparison between natural and residual alkaline materials
Abstract
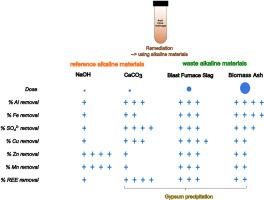
Acid mine drainage (AMD) is a well-known source of toxic trace metals in freshwaters. Traditional passive treatment systems rely on AMD neutralization with limestone and removal of most common toxic transition metals such as Cu and Zn with little attention to rare earth elements (REE). Alkaline waste materials now receive increasing attention as low cost AMD treatment alternatives in the circular economy. This study was set up to identify the efficiency of alkaline waste materials remediating AMD and scavenging REE in addition to other toxic trace elements. An AMD sample was collected from a lixiviate coming from pyrite heaps in the Iberian Pyrite Belt (pH = 1.8, 30 μM ∑REY). The sample was treated with either blast furnace slag (BFS) generated during smelting of iron ore in a blast furnace or biomass ashes (BA) derived from combustion of biomass, thereby using analytical grade CaCO3, and NaOH as reference products. The batch alkalinization experiments were conducted by adding each alkaline material at an amount to obtain an equal pH to ≈6.5. The required amounts of the products were NaOH < CaCO3<BFS < BA in line with their acid neutralizing capacities. The largest removal of sulfate from water was obtained in the CaCO3 treatment suggesting gypsum precipitation which was lower with BA and BFS and virtually absent with NaOH, these trends were confirmed by SEM-EDX and XRD. Both BFS and BA removed more Fe than CaCO3 and NaOH. The REE elements were well removed by all treatments (>99%) and the remaining REE concentrations in the solutions were clearly lower than values for Cu and Zn. The Zn and Cu removals were not consistently high enough (except with NaOH) to meet environmental limits in the discharge waters. The largest efficiency for REE removals was obtained with CaCO3. Indirect evidence here suggests that gypsum is a better host for the trivalent REE than Fe(III) minerals in the precipitates. The ionic radii of trivalent REE are more similar to Ca2+ than to Fe3+, explaining the better potential of gypsum as REE host. This study showed also the potential of BFS as alkaline agent for the remediation of AMD in terms of its higher alkalinity generation potential as compared to BA, thus making BA less promising than BFS.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


