Analgesic switching in chronic users of dextropropoxyphene in France
Abstract
Background
The combination dextropropoxyphene/paracetamol (DXP/P) was the most prescribed opioid analgesic until its withdrawal in 2011.
Objectives
This study investigated dispensations of analgesics in chronic users of DXP/P during the 18 months following its withdrawal.
Methods
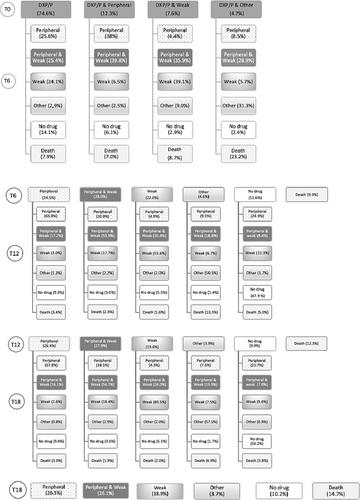
A cross-sectional study repeated yearly was conducted by using the French reimbursement database from 2006 to 2015. Chronic DXP/P users were defined as patients who received at least 40 boxes of DXP/P in the year prior to withdrawal. Data on analgesic dispensing were analyzed at DXP/P withdrawal (T0) and then every 6 months for 18 months.
Results
A total of 63 671 subjects had a DXP/P reimbursement in the year prior to its discontinuation, of whom 7.1% were identified as chronic users (mean age: 71.5 years, women: 68.7%). Among the patients taking DXP/P alone at T0 (74.6%), one fourth switched to a peripheral analgesic, one fourth to a combination of peripheral analgesic/opioid, one fourth to another opioid, and the others mainly discontinued their treatment (14.1%) or died. During the following 12 months, most of the subjects taking only peripheral analgesics continued this treatment, while half of the subjects with a combination of opioid/peripheral analgesic or taking only an analgesic remained on this type of treatment.
Conclusion
Eighteen months after DXP/P withdrawal, more than 10% of patients stopped taking an analgesic. Vigilance is required regarding any change in analgesics by regularly reassessing patients' pain and, in the case of opioid treatments, by monitoring the risk of use disorders.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


