Canonical transient receptor potential channel 1 aggravates myocardial ischemia-and-reperfusion injury by upregulating reactive oxygen species
Abstract
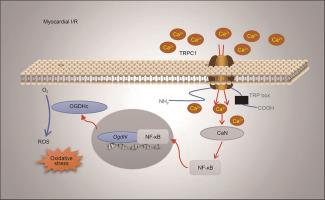
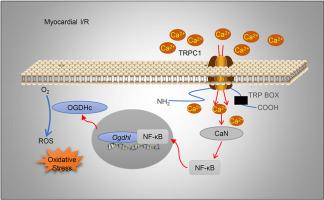
The canonical transient receptor potential channel (TRPC) proteins form Ca2+-permeable cation channels that are involved in various heart diseases. However, the roles of specific TRPC proteins in myocardial ischemia/reperfusion (I/R) injury remain poorly understood. We observed that TRPC1 and TRPC6 were highly expressed in the area at risk (AAR) in a coronary artery ligation induced I/R model. Trpc1−/− mice exhibited improved cardiac function, lower serum Troponin T and serum creatine kinase level, smaller infarct volume, less fibrotic scars, and fewer apoptotic cells after myocardial-I/R than wild-type or Trpc6−/− mice. Cardiomyocyte-specific knockdown of Trpc1 using adeno-associated virus 9 mitigated myocardial I/R injury. Furthermore, Trpc1 deficiency protected adult mouse ventricular myocytes (AMVMs) and HL-1 cells from death during hypoxia/reoxygenation (H/R) injury. RNA-sequencing-based transcriptome analysis revealed differential expression of genes related to reactive oxygen species (ROS) generation in Trpc1−/− cardiomyocytes. Among these genes, oxoglutarate dehydrogenase-like (Ogdhl) was markedly downregulated. Moreover, Trpc1 deficiency impaired the calcineurin (CaN)/nuclear factor-kappa B (NF-κB) signaling pathway in AMVMs. Suppression of this pathway inhibited Ogdhl upregulation and ROS generation in HL-1 cells under H/R conditions. Chromatin immunoprecipitation assays confirmed NF-κB binding to the Ogdhl promoter. The cardioprotective effect of Trpc1 deficiency was canceled out by overexpression of NF-κB and Ogdhl in cardiomyocytes. In conclusion, our findings reveal that TRPC1 is upregulated in the AAR following myocardial I/R, leading to increased Ca2+ influx into associated cardiomyocytes. Subsequently, this upregulates Ogdhl expression through the CaN/NF-κB signaling pathway, ultimately exacerbating ROS production and aggravating myocardial I/R injury.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


