Incorporating Cyano Groups to a Conjugated Polymer Based on Double B←N-Bridged Bipyridine Units for Unipolar n-Type Organic Field-Effect Transistors
引用次数: 2
Abstract
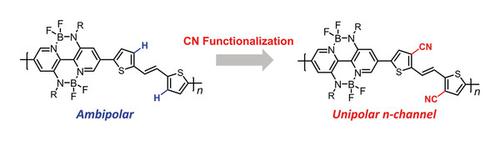
Abstract The development of n-type semiconductors lags far behind that of their p-type counterparts, demonstrating the exploration of exclusive n-type π-conjugated polymers is significant. The double B←N-bridged bipyridine (BNBP)-based polymers P-BNBP-TVT containing (E)-1,2-di(thiophen-2-yl)ethene (TVT) previously reported exhibits ambipolar character because of the electron-rich nature. Herein, we incorporated strong electron-withdrawing cyano groups into the 3,3′-positions of the TVT moiety to a copolymer P-BNBP-2CNTVT to develop n-type π-conjugated polymers. The LUMO/HOMO energy levels of P-BNBP-2CNTVT are −3.80/−5.95 eV, respectively, which are ~0.4 eV lower than that of P-BNBP-TVT without cyano groups. Unsurprisingly, compared with ambipolar P-BNBP-TVT, the organic field-effect transistors (OFETs) based on P-BNBP-2CNTVT showed unipolar n-type characteristics with an electron mobility of 0.026 cm2 · V−1 · s−1 and a lower threshold voltage of ~25 V as well as high I on/I off of ~105. This study demonstrates that organoboron π-conjugated polymers could be regarded as a tool for constructing exclusive n-type semiconducting polymers used in OFETs.
单极n型有机场效应晶体管中基于双B←n桥联吡啶单元的氰基共轭聚合物
n型半导体的发展远远落后于p型半导体,这表明探索n型π共轭聚合物具有重要意义。先前报道的含有(E)-1,2-二(噻吩-2-基)乙烯(TVT)的双B←n桥联吡啶(BNBP)基聚合物P-BNBP-TVT由于富电子性质而具有双极性特征。本文将强吸电子氰基引入到共聚物P-BNBP-2CNTVT的3,3 '位,制备了n型π共轭聚合物。P-BNBP-2CNTVT的LUMO/HOMO能级分别为−3.80/−5.95 eV,比不含氰基的P-BNBP-TVT低~0.4 eV。不出所料,与双极性P-BNBP-TVT相比,基于P-BNBP-2CNTVT的有机场效应晶体管(ofet)表现出单极n型特性,电子迁移率为0.026 cm2·V−1·s−1,阈值电压较低,为~25 V, I /I关高,为~105。该研究表明,有机硼π共轭聚合物可以作为构建专用于ofet的n型半导体聚合物的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


