Exploring Indeno[2,1- c ]fluorene Antiaromatics with Unsymmetrical Disubstitution and Balanced Ambipolar Charge-Transport Properties
引用次数: 2
Abstract
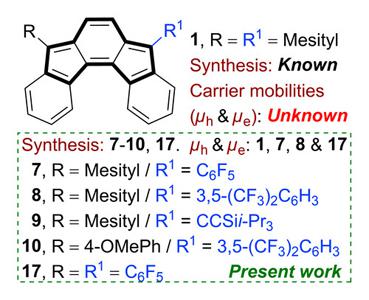
Unsymmetrically disubstituted antiaromatic indenofluorene (IF), in comparison to aromatic pentacene counterpart with unsymmetrical disubstitution, was rare in literature until our recent report on indeno[1,2-b]fluorene and indeno[2,1-a]fluorene. Described herein is straightforward access to unsymmetrically disubstituted indeno[2,1-c]fluorenes bearing mesityl at one apical carbon and C6F5, 3,5-(CF3)2C6H3, and CCSii-Pr3 at the other apical carbon, including 4-methoxyphenyl/3,5-(CF3)2C6H3 push/pull substitution at the apical carbons with appreciable orbital density and a previously unknown symmetrically C6F5-disubstituted [2,1-c]IF. The electronic properties of the unsymmetrical derivatives lie halfway in between the two symmetrical counterparts, while the 4-methoxyphenyl derivative showed the smallest HOMO-LUMO energy gap and near-infrared absorption with intramolecular charge transfer character. Single-crystal analyses showed 1D-columnar stacks for the unsymmetrical motif with the C6F5 units co-facially π-stacked with the IF core, whereas symmetrically C6F5-disubstituted [2,1-c]IF, with a low-lying LUMO, showed intermolecular π-π stacks between the IFs core that resulted in good electron mobility (μe = 8.66 × 10−3 cm2 V−1 s−1) under space charge limited current measurements. Importantly, balanced ambipolar charge-transport behaviour could be extracted for an IF series with symmetrical/unsymmetrical substitutions, in comparison to its π-contracted pentalene congener.
具有不对称二取代和平衡双极电荷输运性质的茚并[2,1-c]芴反芳烃的探索
在我们最近关于茚并[1,2-b]芴和茚并[2,1-a]芴的报道之前,与具有不对称二取代的芳香并五苯对应物相比,不对称二取代反芳香茚并芴(IF)在文献中是罕见的。本文所描述的是直接获得在一个顶端碳上具有均三甲苯和在另一个顶端炭上具有C6F5、3,5-(CF3)2C6H3和CCSii-Pr3的非对称二取代茚并[2,1-c]芴,包括在具有可观轨道密度的顶端碳上的4-甲氧基苯基/3,5-(CF3。不对称衍生物的电子性质介于两个对称衍生物之间,而4-甲氧基苯基衍生物表现出最小的HOMO-LUMO能隙和具有分子内电荷转移特性的近红外吸收。单晶分析显示,不对称基序的1D柱状堆叠,C6F5单元与IF核共面π-堆叠,而对称的C6F5二取代[2,1-c]IF,具有较低的LUMO,显示IF核之间的分子间π-π堆叠,在空间电荷限制电流测量下产生良好的电子迁移率(μe=8.66×10−3 cm2 V−1 s−1)。重要的是,与π-收缩的亚戊烯同系物相比,具有对称/不对称取代的IF系列可以提取平衡的双极电荷传输行为。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


