Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells
引用次数: 0
Abstract
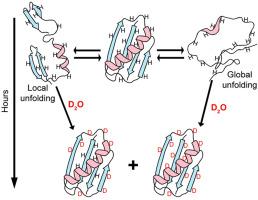
We review the use of nuclear magnetic resonance (NMR) spectroscopy to assess the exchange of amide protons for deuterons (HDX) in efforts to understand how high concentration of cosolutes, especially macromolecules, affect the equilibrium thermodynamics of protein stability. HDX NMR is the only method that can routinely provide such data at the level of individual amino acids. We begin by discussing the properties of the protein systems required to yield equilibrium thermodynamic data and then review publications using osmolytes, sugars, denaturants, synthetic polymers, proteins, cytoplasm and in cells.
利用核磁共振检测氢-氘交换,定量蛋白质在体外和细胞中溶质和拥挤条件下的稳定性
我们回顾了核磁共振(NMR)光谱的应用,以评估酰胺质子与氘核(HDX)的交换,以了解高浓度的辅质,特别是大分子,如何影响蛋白质稳定性的平衡热力学。HDX核磁共振是唯一的方法,可以常规提供这样的数据在单个氨基酸水平。我们首先讨论产生平衡热力学数据所需的蛋白质系统的性质,然后回顾使用渗透物,糖,变性剂,合成聚合物,蛋白质,细胞质和细胞中的出版物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Magnetic Resonance Letters
Analytical Chemistry, Spectroscopy, Radiology and Imaging, Biochemistry, Genetics and Molecular Biology (General)
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


