An extension of Free's extraction isotherm for the solvent extraction of cations using acidic extractants
Abstract
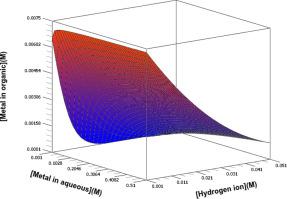
A simple theoretical model has been developed for the solvent extraction of cations with different oxidation states varying from +1 to +4 using acidic extractants. The basic assumption behind this theoretical model is that the cationic species (Mn+) will exist in ionic form and not form any complexes in the aqueous phase. A direct relationship between the concentration of metal ions in the aqueous phase and that in the organic phase was developed for trivalent and tetravalent cationic species. Using this model, the distribution isotherms were plotted for a given set of conditions. The effect of pH and equilibrium constant on the distribution isotherms were represented in three-dimensional form for trivalent and tetravalent cationic species. Consistent with expectations, the results suggest that with increasing pH and an increasing equilibrium constant one finds an enhancement of the extraction of cationic species for a given set of conditions. The calculations were performed in the pH range of 1 through 3 because for higher pH values, the pH shift accompanying the extraction is significant and thereby affects the mass balance equations. A comparison of the distribution isotherms shows that the total metal ion concentration to extractant concentration ratio determines the distribution behavior of metal ions with different valency. At low total metal ion concentration to extractant concentration ratio, the distribution coefficient was higher for higher oxidation state cations under similar experimental conditions, and this trend started to reverse when the total metal ion concentration to extractant concentration ratio was increased.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


