Microphysiological systems in the evaluation of hematotoxicities during drug development
IF 4.6
引用次数: 4
Abstract
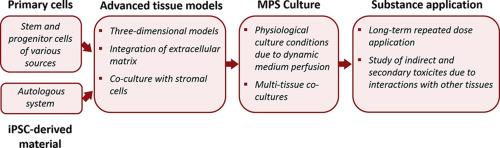
Microphysiological systems are progressively entering the pharmaceutical industry, and various systems have already proven to be highly valuable at different stages of the drug development process. The field of hematotoxicity research has so far received only minor attention, even though microphysiological systems might provide key benefits over current assays. In this review, we will highlight the need for more complex human in vitro assays, and how emerging technologies such as microphysiological systems present novel solutions for the study of adverse hematologic effects.
药物开发过程中评估血液毒性的微生理系统
微生理系统正逐步进入制药行业,各种系统已被证明在药物开发过程的不同阶段具有很高的价值。迄今为止,血液毒性研究领域只受到很少的关注,尽管微生理系统可能比目前的检测方法提供关键的好处。在这篇综述中,我们将强调对更复杂的人体体外检测的需求,以及微生理系统等新兴技术如何为血液不良反应的研究提供新的解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current opinion in toxicology
Toxicology, Biochemistry
CiteScore
8.50
自引率
0.00%
发文量
0
审稿时长
64 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


