Safety and effectiveness of eculizumab in Japanese patients with generalized myasthenia gravis: Analysis of 1-year postmarketing surveillance
Abstract
Introduction
Eculizumab, a terminal complement protein C5 inhibitor, is approved in Japan for the treatment of patients with anti-acetylcholine receptor antibody-positive (AChR Ab+) generalized myasthenia gravis (gMG) that is difficult to control with plasmapheresis or high-dose intravenous immunoglobulin therapy.
Methods
This analysis of mandatory postmarketing surveillance in Japan assessed the safety and effectiveness of eculizumab in patients with AChR Ab+ gMG who had completed case-report forms at 26 wk after eculizumab initiation up to the cutoff date of April 2021. Changes from baseline were assessed for Myasthenia Gravis-Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) total scores overall, and MG-ADL scores in patient subgroups according to sex, age at diagnosis and baseline, and baseline disease severity. The change in concomitant corticosteroid use was also evaluated.
Results
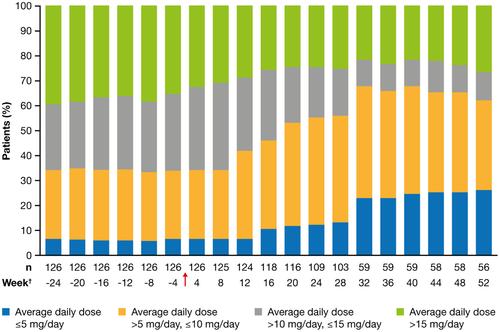
Data were available for 134 adults (67.2% female; mean age 51.9 y); the effectiveness-analysis set comprised 126 patients. After 26 wk, 78% of patients were continuing eculizumab treatment. Adverse drug reactions were reported by 49 patients (37%) (most frequently headache [n = 10]). Improvements in MG-ADL scores were seen regardless of sex; age at diagnosis (<50/≥50 y); baseline age (18 to <40/≥40 to <65/≥65 y); Myasthenia Gravis Foundation of America disease classification (IIa/IIb/IIIa/IIIb/IVa/IVb/V); or baseline MG-ADL score (<6/≥6). Of patients receiving corticosteroids, the proportion receiving low doses (average ≤5 mg/d) increased from 7.0% before eculizumab initiation to 26.4% by Week 52.
Conclusion
Eculizumab was well tolerated and effective in treating AChR Ab+ gMG across a broad spectrum of adult Japanese patients with difficult-to-control gMG.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


