Systematic review and network meta-analysis comparing ofatumumab with other disease-modifying therapies available in Japan for the treatment of patients with relapsing multiple sclerosis
Abstract
Objective
No head-to-head clinical trials have compared ofatumumab with other disease-modifying therapies (DMTs) available in Japan for patients with relapsing multiple sclerosis (RMS). In this study, a network meta-analysis (NMA) was conducted to compare the efficacy of ofatumumab to other DMTs currently available in Japan for the treatment of patients with RMS.
Methods
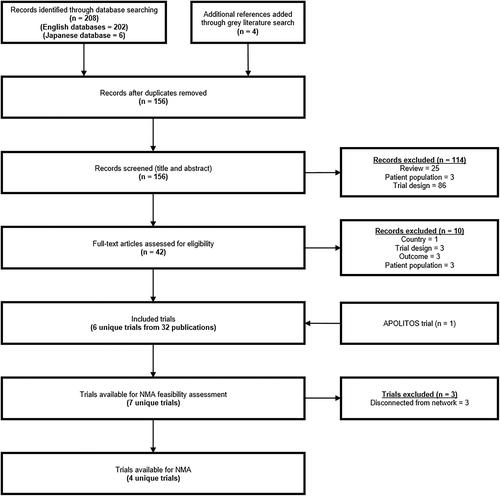
Systematic searches were conducted in biomedical databases from inception to June 2020 to identify randomized controlled trials. Only English- and Japanese-language publications describing studies conducted in Japan were included. Trials with sufficiently similar study and patient characteristics were included in a Bayesian NMA. A sensitivity analysis was conducted to explore the impact of potential sources of uncertainty.
Results
Four trials, each comparing a DMT with placebo in a ≥50% Japanese population, were sufficiently similar that comparative efficacy could be assessed for annualized relapse rate (ARR). Ofatumumab numerically reduced ARR compared with fingolimod (rate ratio [RR]: 0.84, 95% credible interval [CrI]: 0.20–3.39), dimethyl fumarate (RR: 0.61, 95% CrI: 0.16–2.30), and placebo (RR: 0.41, 95% CrI: 0.12–1.39), but not natalizumab (RR: 1.33, 95% CrI: 0.33–5.45). In a subgroup analysis of Japanese patients only, ofatumumab reduced relapses compared with all other treatments including natalizumab. These results were limited by the lack of studies reporting direct comparisons between included treatments and by heterogenous reporting of outcome data.
Conclusion
These findings, although limited by the paucity of evidence for Japanese patients, suggest that monoclonal antibody therapies (ie, natalizumab and ofatumumab) may provide improved efficacy compared with other DMTs available in Japan for patients with RMS.

| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


