PL‐101‐WK, a novel tryptophan‐ and lysine‐rich peptide with antimicrobial activity against Staphylococcus aureus
IF 1.5
4区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Designing short antimicrobial peptides (AMPs), which are active against drug‐resistant bacteria, is a promising way to find new therapeutic agents. In this research, a novel short AMP, PL‐101‐WK, was designed based on PL‐101 (a derivative of plicatamide). Here, the substitution of Phe and His with Trp and Lys was considered. The antimicrobial activity and physicochemical properties of PL‐101‐WK were compared with PL‐101 by in silico analysis. The antimicrobial activity in the presence or absence of NaCl concentration, thermal stability, hemolytic activity, and selectivity of the peptides were determined. By substitution of Lys and Trp residues, positive charge, in vitro stability, and hydrophilicity of PL‐101‐WK were raised compared to the template. PL‐101‐WK had the best minimum inhibitory concentration (MIC) value of 64 μg/ml against Staphylococcus aureus strains, which showed at least 16‐fold reduction when compared to the values of PL‐101. The MICs of PL‐101‐WK were retained toward S. aureus strains at physiological salt concentration. While PL‐101‐WK did not display acceptable thermal stability, it had desirable selectivity against bacteria. The maximum hemolytic activity of PL‐101‐WK was 1.65% at 512 μg/ml. Taken together, increasing positive charge and the presence of Trp residues were enhanced the potential of antibacterial activity of PL‐101.
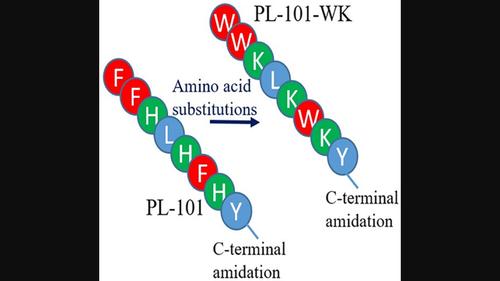
PL‐101‐WK,一种新型富含色氨酸和赖氨酸的肽,对金黄色葡萄球菌具有抗菌活性
设计对耐药细菌具有活性的短抗菌肽(AMPs)是寻找新的治疗药物的一种很有前途的方法。在这项研究中,基于PL‐101 (plicatamide的衍生物)设计了一种新的短AMP PL‐101‐WK。这里考虑用Trp和Lys取代Phe和His。通过硅分析比较了PL‐101‐WK与PL‐101的抑菌活性和理化性质。测定了在NaCl浓度存在或不存在情况下的抗菌活性、热稳定性、溶血活性和选择性。通过替换赖氨酸和色氨酸残基,与模板相比,PL‐101‐WK的正电荷、体外稳定性和亲水性都得到了提高。PL‐101‐WK对金黄色葡萄球菌的最低抑菌浓度(MIC)为64 μg/ml,比PL‐101降低了至少16倍。在生理盐浓度下,PL‐101‐WK对金黄色葡萄球菌的mic保持不变。虽然PL‐101‐WK没有表现出可接受的热稳定性,但它对细菌具有理想的选择性。在512 μg/ml浓度下,PL‐101‐WK的最大溶血活性为1.65%。综上所述,正电荷的增加和色氨酸残基的存在增强了PL‐101的抗菌活性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Peptide Science
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
5.20
自引率
4.20%
发文量
36
期刊介绍:
The aim of Peptide Science is to publish significant original research papers and up-to-date reviews covering the entire field of peptide research. Peptide Science provides a forum for papers exploring all aspects of peptide synthesis, materials, structure and bioactivity, including the use of peptides in exploring protein functions and protein-protein interactions. By incorporating both experimental and theoretical studies across the whole spectrum of peptide science, the journal serves the interdisciplinary biochemical, biomaterials, biophysical and biomedical research communities.
Peptide Science is the official journal of the American Peptide Society.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


