Network Biology Approaches to Uncover Therapeutic Targets Associated with Molecular Signaling Pathways from circRNA in Postoperative Cognitive Dysfunction Pathogenesis
Abstract
Abstract
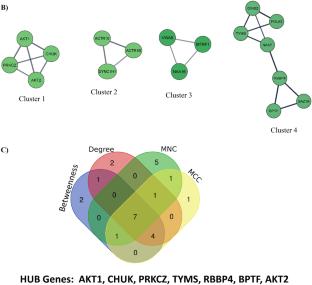
Postoperative cognitive dysfunction (POCD) is a cognitive deterioration and dementia that arise after a surgical procedure, affecting up to 40% of surgery patients over the age of 60. The precise etiology and molecular mechanisms underlying POCD remain uncovered. These reasons led us to employ integrative bioinformatics and machine learning methodologies to identify several biological signaling pathways involved and molecular signatures to better understand the pathophysiology of POCD. A total of 223 differentially expressed genes (DEGs) comprising 156 upregulated and 67 downregulated genes were identified from the circRNA microarray dataset by comparing POCD and non-POCD samples. Gene ontology (GO) analyses of DEGs were significantly involved in neurogenesis, autophagy regulation, translation in the postsynapse, modulating synaptic transmission, regulation of the cellular catabolic process, macromolecule modification, and chromatin remodeling. Pathway enrichment analysis indicated some key molecular pathways, including mTOR signaling pathway, AKT phosphorylation of cytosolic targets, MAPK and NF-κB signaling pathway, PI3K/AKT signaling pathway, nitric oxide signaling pathway, chaperones that modulate interferon signaling pathway, apoptosis signaling pathway, VEGF signaling pathway, cellular senescence, RANKL/RARK signaling pathway, and AGE/RAGE pathway. Furthermore, seven hub genes were identified from the PPI network and also determined transcription factors and protein kinases. Finally, we identified a new predictive drug for the treatment of SCZ using the LINCS L1000, GCP, and P100 databases. Together, our results bring a new era of the pathogenesis of a deeper understanding of POCD, identified novel therapeutic targets, and predicted drug inhibitors in POCD.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


