Nicholas J Chargo, Jonathan D Schepper, Naoimy Rios-Arce, Ho Jun Kang, Joseph D Gardinier, Narayanan Parameswaran, Laura R McCabe
{"title":"Lactobacillus Reuteri 6475 Prevents Bone Loss in a Clinically Relevant Oral Model of Glucocorticoid-Induced Osteoporosis in Male CD-1 Mice","authors":"Nicholas J Chargo, Jonathan D Schepper, Naoimy Rios-Arce, Ho Jun Kang, Joseph D Gardinier, Narayanan Parameswaran, Laura R McCabe","doi":"10.1002/jbm4.10805","DOIUrl":null,"url":null,"abstract":"<p>Glucocorticoids (GCs) are commonly used anti-inflammatory medications with significant side effects, including glucocorticoid-induced osteoporosis (GIO). We have previously demonstrated that chronic subcutaneous GC treatment in mice leads to gut barrier dysfunction and trabecular bone loss. We further showed that treating with probiotics or barrier enhancers improves gut barrier function and prevents GIO. The overall goal of this study was to test if probiotics could prevent GC-induced gut barrier dysfunction and bone loss in a clinically relevant oral-GC model of GIO. Eight-week-old male CD-1 mice were treated with vehicle or corticosterone in the drinking water for 4 weeks and administered probiotics <i>Lactobacillus reuteri</i> ATCC 6475 (LR 6475) or VSL#3 thrice weekly via oral gavage. As expected, GC treatment led to significant gut barrier dysfunction (assessed by measuring serum endotoxin levels) and bone loss after 4 weeks. Serum endotoxin levels significantly and negatively correlated with bone volume. Importantly, LR 6475 treatment effectively prevented both GC-induced increase in serum endotoxin and trabecular bone loss. VSL#3 had intermediate results, not differing from either control or GC-treated animals. GC-induced reductions in femur length, cortical thickness, and cortical area were not affected by probiotic treatment. Taken together, these results are the first to demonstrate that LR 6475 effectively prevents the detrimental effects of GC treatment on gut barrier, which correlates with enhanced trabecular bone health in an oral mouse model of GIO. © 2023 The Authors. <i>JBMR Plus</i> published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.</p>","PeriodicalId":14611,"journal":{"name":"JBMR Plus","volume":"7 12","pages":""},"PeriodicalIF":3.4000,"publicationDate":"2023-08-13","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://asbmr.onlinelibrary.wiley.com/doi/epdf/10.1002/jbm4.10805","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"JBMR Plus","FirstCategoryId":"1085","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/jbm4.10805","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q2","JCRName":"ENDOCRINOLOGY & METABOLISM","Score":null,"Total":0}
引用次数: 0
Abstract
Glucocorticoids (GCs) are commonly used anti-inflammatory medications with significant side effects, including glucocorticoid-induced osteoporosis (GIO). We have previously demonstrated that chronic subcutaneous GC treatment in mice leads to gut barrier dysfunction and trabecular bone loss. We further showed that treating with probiotics or barrier enhancers improves gut barrier function and prevents GIO. The overall goal of this study was to test if probiotics could prevent GC-induced gut barrier dysfunction and bone loss in a clinically relevant oral-GC model of GIO. Eight-week-old male CD-1 mice were treated with vehicle or corticosterone in the drinking water for 4 weeks and administered probiotics Lactobacillus reuteri ATCC 6475 (LR 6475) or VSL#3 thrice weekly via oral gavage. As expected, GC treatment led to significant gut barrier dysfunction (assessed by measuring serum endotoxin levels) and bone loss after 4 weeks. Serum endotoxin levels significantly and negatively correlated with bone volume. Importantly, LR 6475 treatment effectively prevented both GC-induced increase in serum endotoxin and trabecular bone loss. VSL#3 had intermediate results, not differing from either control or GC-treated animals. GC-induced reductions in femur length, cortical thickness, and cortical area were not affected by probiotic treatment. Taken together, these results are the first to demonstrate that LR 6475 effectively prevents the detrimental effects of GC treatment on gut barrier, which correlates with enhanced trabecular bone health in an oral mouse model of GIO. © 2023 The Authors. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
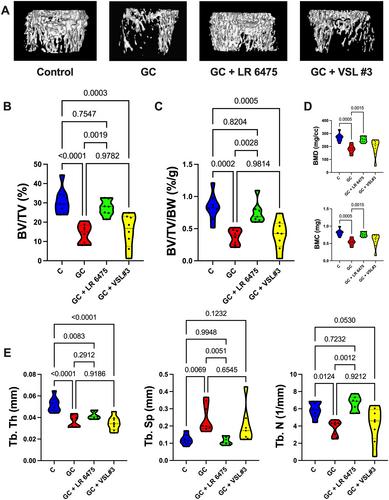
罗伊氏乳杆菌6475在糖皮质激素诱导的雄性CD‐1小鼠骨质疏松症的临床相关口腔模型中预防骨质流失
糖皮质激素(GC)是常用的抗炎药,具有显著的副作用,包括糖皮质激素诱导的骨质疏松症(GIO)。我们之前已经证明,小鼠的慢性皮下GC治疗会导致肠道屏障功能障碍和骨小梁丢失。我们进一步表明,用益生菌或屏障增强剂治疗可以改善肠道屏障功能并预防GIO。本研究的总体目标是在临床相关的GIO口服GC模型中测试益生菌是否可以预防GC诱导的肠道屏障功能障碍和骨丢失。8周龄雄性CD-1小鼠在饮用水中用载体或皮质酮治疗4周 周,并通过口服管饲每周三次给予益生菌罗伊氏乳杆菌ATCC 6475(LR 6475)或VSL#3。正如预期的那样,GC治疗导致了显著的肠道屏障功能障碍(通过测量血清内毒素水平来评估)和4天后的骨丢失 周。血清内毒素水平与骨体积呈显著负相关。重要的是,LR 6475治疗有效地防止了GC诱导的血清内毒素增加和骨小梁丢失。VSL#3具有中等结果,与对照或GC处理的动物没有差异。GC诱导的股骨长度、皮质厚度和皮质面积的减少不受益生菌治疗的影响。总之,这些结果首次证明LR 6475有效预防GC治疗对肠道屏障的有害影响,这与GIO口服小鼠模型中骨小梁健康的增强有关。©2023作者。由Wiley Periodicals LLC代表美国骨与矿物研究学会出版的JBMR Plus。
本文章由计算机程序翻译,如有差异,请以英文原文为准。


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


