Yttrium nitrate promoted selective cyanoethylation of amines
Abstract
The catalyst Y(NO3)3, 6H2O exhibited remarkable activity in the aza-Michael addition of various aromatic and aliphatic amines with acrylonitrile at ambient temperature in a protic solvent. The method is selective for the monocyanoethylation of primary aromatic amines, aliphatic secondary amines, and sterically hindered aliphatic amines. Phenols and active methylene compounds do not undergo cyanoethylation. Thiophenol, in the presence of yttrium nitrate, promotes the polymerization of acrylonitrile. The water solubility and high catalyst stability make the process of removing the catalyst from the product easy. Direct aqueous workup of the reaction mixture could lead to the isolation of cyanoethylation products up to 99.9% purity.
Graphical Abstract
-
Selective mono-cyanoethylation at the primary aromatic amine, especially electron-rich aromatic amines
-
Excellent regio-selectivity in the presence of Carbon and Oxygen nucleophiles
-
The reported condition could be used to polymerize acrylonitrile in the presence of thiophenol.
-
Reaction is facile for electron-rich aromatic amine.
-
Highly sensitive to the steric effect at the nitrogen center
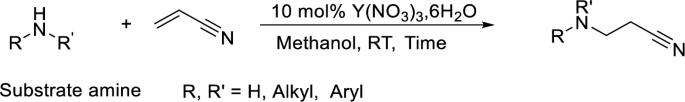

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


