Design, Development, In Silico, and In Vitro Characterization of Camptothecin-Loaded Mixed Micelles: In Vitro Testing of Verapamil and Ranolazine for Repurposing as Coadjuvant Therapy in Cancer
Abstract
Abstract
Purpose
Camptothecin has poor solubility, high systemic toxicity, and intrinsic structural instability. To deal with these challenges, present research aimed to develop camptothecin-loaded mixed micelles (CPT MMs) using TPGS and Pluronic® F108 copolymers. Furthermore, our research aimed to test in vitro anticancer activities of non-micellar verapamil and ranolazine for repurposing as coadjuvant therapy with CPT MMs in cancer.
Methods
CPT MMs were fabricated by solvent evaporation method and optimized using 32 full factorial design. CPT MMs were characterized for % entrapment efficiency (%EE), mean particle size (MPS), zeta potential, surface morphology, % drug loading capacity (%DLC), in vitro drug release, and in vitro cytotoxicity and cell cycle arresting behaviors.
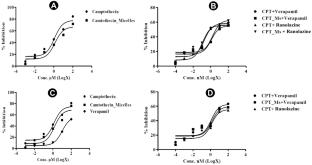
Result
The in silico studies revealed decent camptothecin interaction with a cavity of mixed micelles (MMs). CPT MMs composition (H5) is considered optimum based on %EE (94.92 ± 2.46%), MPS (136.9 ± 1.71 nm), zeta potential (− 22.9 ± 0.87 mV), and %DLC (1.810 ± 0.02%). TEM image shows self-assembled micelles with spherical shape. CPT MMs showed sustained release profile. The drug-excipient compatibility study revealed no primary incompatibilities. The CPT MMs showed moderately higher IC50 values than camptothecin against A549 and B16F10 cells. The non-micellar verapamil and ranolazine when combined with CPT MMs at lower concentrations have resulted in substantially higher cytotoxicity. Whereas, the CPT MMs + ranolazine combination has shown higher cell cycle arresting behavior than CPT MMs + verapamil combination.
Conclusion
Elaborative and molecular mechanism–based studies are further needed to validate the repurposing potential of non-micellar verapamil and ranolazine as coadjuvant with CPT MMs in cancer.
Graphical Abstract

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


