Molecular Layer Deposition (MLD) of a Blocked Mercapto Silane on Precipitated Silica
引用次数: 0
Abstract
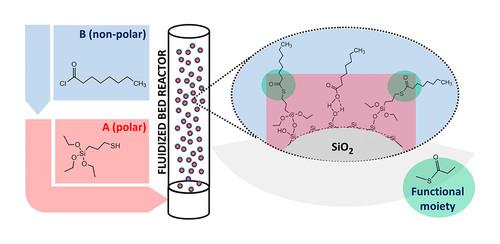
Chemically modified silica is widely used as a reinforcing filler in elastomers. The modification is generally done in situ while preparing the rubber. However, in order to increase the efficiency and facilitate the mixing process, the silica can be pre-treated by a 2-step molecular layer deposition. The precursors for the modification are 3-mercaptopropyl-triethoxysilane (MPTES) and octanoyl chloride (OC) to react with MPTES and form a blocked silane. The precipitated silica nanofiller was successfully treated with MPTES and showed a self-limiting behavior: saturation occurred at 2.7%. Furthermore, DRIFTS (diffuse reflectance infrared Fourier transform spectroscopy) analysis confirmed the successful deposition of MPTES on the silica surface by showing the -SH peak that appeared after the reaction of MPTES and silica. In the second step, OC was introduced to form a thioester on the surface of the MPTES-treated silica, controlling the reactivity of the mercapto group from MPTES by blocking it to prevent a negative influence on the processing behavior of the rubber. Thermogravimetric analysis (TGA), Fourier-transform infrared spectroscopy, and X-ray photoelectron spectroscopy (XPS) analytical results confirmed the deposition of the blocked mercapto silane on the silica. TGA results demonstrated the self-limiting behavior of OC, and DRIFTS and XPS proved the thioester formation. A thioester peak after the 2nd reaction step with OC appeared. At the same time, the disappearance of the -SH signal from the MPTES was observed, indicating the formation of the blocked mercapto silane structure. Transmission electron microscopy results showed that the treated silica has a well-distributed carbon and sulfur deposition after MPTES/OC treatment.
封闭巯基硅烷在沉淀二氧化硅上的分子层沉积
化学改性二氧化硅被广泛用作弹性体中的增强填料。改性通常在制备橡胶时原位进行。然而,为了提高效率并促进混合过程,二氧化硅可以通过两步分子层沉积进行预处理。改性的前体是3-巯基丙基-三乙氧基硅烷(MPTES)和辛酰氯(OC),与MPTES反应形成封端硅烷。沉淀的二氧化硅纳米填料用MPTES成功处理,并显示出自限制行为:饱和发生在2.7%。此外,DRIFTS(漫反射红外傅立叶变换光谱)分析通过显示MPTES和二氧化硅反应后出现的-SH峰,证实了MPTES在二氧化硅表面的成功沉积。在第二步中,引入OC以在MPTES处理的二氧化硅的表面上形成硫酯,通过阻断巯基来控制巯基与MPTES的反应性,以防止对橡胶的加工行为产生负面影响。热重分析(TGA)、傅立叶变换红外光谱和X射线光电子能谱(XPS)分析结果证实了封端巯基硅烷在二氧化硅上的沉积。TGA结果证明了OC的自限制行为,DRIFTS和XPS证明了硫酯的形成。在与OC的第二反应步骤之后出现硫酯峰。同时,观察到来自MPTES的-SH信号消失,表明形成了封闭的巯基硅烷结构。透射电子显微镜结果表明,经MPTES/OC处理后的二氧化硅具有均匀分布的碳和硫沉积。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


