Safety and effectiveness of dupilumab in the real-world treatment of atopic dermatitis in Japan: 1-year interim analysis from a post-marketing surveillance
Abstract
Objectives
Atopic dermatitis (AD) is a common chronic inflammatory skin disorder in Japan. Dupilumab, a fully human monoclonal antibody, targets a shared subunit of the interleukin (IL)-4 and IL-13 receptors. Post-marketing surveillance of the safety and effectiveness of dupilumab in adult AD patients was conducted in Japan, where the drug is also allowed for use in older adolescents (i.e., ≥15 years), and interim results are reported here.
Methods
This observational, multicenter study enrolled Japanese patients with AD who initiated dupilumab between July 2018–June 2020 (UMIN-CTR Trials Registry: UMIN000032807). Baseline demographics, clinical history, medication data and dupilumab safety and effectiveness data were collected.
Results
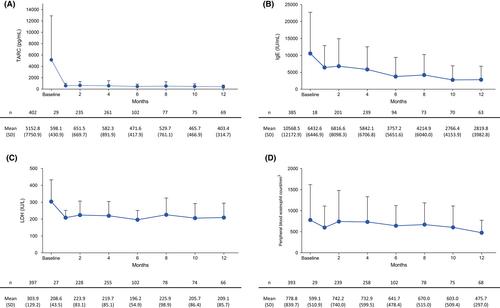
By the data cut-off date of March 26, 2021, information from 600 patients has been collected. All the available safety and 1-year effectiveness data are presented. The mean (standard deviation) age was 42.0 (15.9) years, the majority (69.1%) were male, and asthma was present in 12.2%. Adverse drug reactions (ADRs) were observed in 98 patients (16.4%), including conjunctivitis (n = 40; 6.7%), conjunctivitis allergic (n = 30; 5.0%), blepharitis (n = 5; 0.8%), headache and eye pruritus (n = 4; 0.7% each) and eosinophilia (n = 3; 0.5%). Six patients experienced asthma, all of whom had a history of, or concurrent, asthma. Disease severity improved remarkably at 4 months in most patients, which was maintained up to 1 year.
Conclusion
Dupilumab appears to be a safe and effective treatment for patients aged ≥15 years with moderate-to-severe AD in routine clinical practice in Japan. Dupilumab was well tolerated, with no new safety signals and no new-onset asthma.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


