Improved residue on ignition method for the mass fraction of inorganic impurities of pure organic substances
Abstract
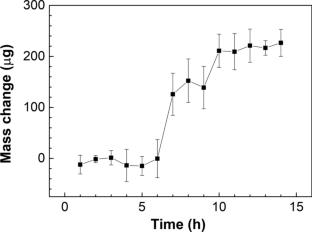
The mass fraction of inorganic impurities of a pure organic substance can be determined by the residue on ignition method and then used to calculate the purity according to mass balance theory. The conventional residue on ignition method is insensitive. In this study, a novel crucible made of annealed aluminum foil was used instead of the conventional crucible. The mass of the annealed aluminum crucible on ignition was repeatable (standard deviation of 1.4 µg). The mass of the annealed aluminum crucible remained stable in the ambient atmosphere because no water adsorbed to the surface of annealed aluminum. When annealed at high temperature, the mass of the aluminum crucible increased owing to the oxidation of aluminum. On subsequent annealing, the amorphous aluminum oxide (Al2O3) film transformed into a γ-Al2O3 film which prevented the inner aluminum from oxidation and adsorption of water. The limit of quantification of the mass of inorganic impurities determined by the residue on ignition method using the annealed aluminum crucible (30 µg) was much lower than that using a platinum crucible (500 µg). When the mass of solute of sodium chloride (NaCl) aqueous solution was in the range 20 µg–200 µg, the recovery of the NaCl mass fraction was in the range 96 %–104 %. The residue on ignition method was applied to eight pure organic substances. The improved residue on ignition method using the annealed aluminum crucible determined the mass fractions of inorganic impurities of the pure organic substances with high sensitivity and accuracy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


