Real-world effectiveness and safety of dupilumab in patients with moderate and severe atopic dermatitis: 2-year experience
Abstract
Objective
Dupilumab has been deemed highly effective for atopic dermatitis (AD). However, there have been no reports performing a combination analysis with hematological data and improvement rates pertaining to the continued use of dupilumab for up to 2 years in real world. In this study, we evaluated the effectiveness and safety of using dupilumab for up to 2 years in 9 patients with AD at our hospital.
Methods
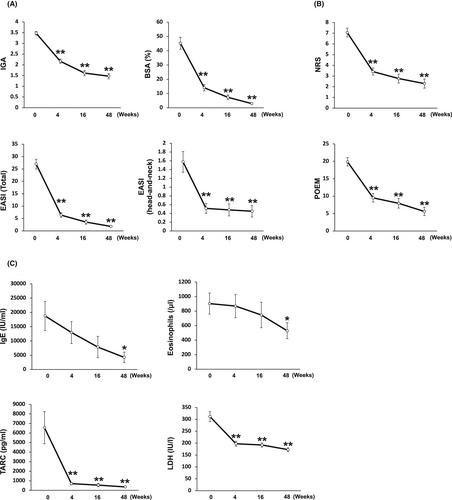
Thirty-six patients with moderate-to-severe AD treated by dupilumab, and 9 of them treated for 2 years. Changes in the severity scoring, pruritus numerical rating scale (NRS), patient-oriented eczema measure (POEM), serum levels of immunoglobulin E (IgE), thymus and activation-regulated chemokine (TARC), eosinophils, and lactate dehydrogenase (LDH) at Week 0, 2, 4, 16, 48, 72, and 96 of those patients were investigated, and we studied features of the patients who had any adverse events (AEs).
Results
Investigator’s global assessment (IGA), eczema area and severity index (EASI), body surface area (BSA), NRS, POEM, and serum levels of LDH were significantly decreased from Week 4 onwards to Week 96 compared with baseline condition. Serum levels of TARC and LDH were significantly decreased from Week 4 onwards to Week 96. Regarding 9 patients who were treated with dupilumab for up to 2 years, serum levels of TARC and eosinophils decreased without statistical significance. The serum levels of IgE significantly decreased at Week 72, 96 compared with the baseline. Regarding as AEs, ocular symptoms were the most frequently observed (15/36, 41.2%), and there were no cases of discontinuation due to AEs.
Conclusions
Treatment with dupilumab was well tolerated and showed improvements in AD for at least 2 years.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


