Nickel ferrite magnetic nanoparticles: evidence for superparamagnetism in smaller size particles
Abstract
Abstract
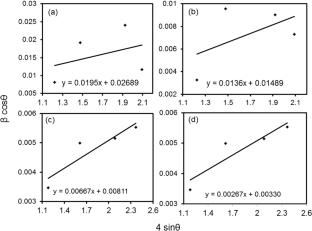
Nickel ferrite magnetic nanoparticles have been synthesized by the co-precipitation method using stable nickel chloride and iron chloride salts with sodium hydroxide as the precipitating agent and polyethylene glycol as the capping agent. Annealing was adopted to get single-phase nickel ferrite nanoparticles and then the samples were characterized by X-ray diffraction, ultraviolet–visible spectroscopy, Fourier transform infrared spectroscopy, photoluminescence spectroscopy, transmission electron microscopy, vibrating sample magnetometer, electron paramagnetic resonance, and cyclic voltammetry. An X-ray diffraction study confirmed the single-phase, cubic inverse spinel crystal structure of the samples. With varying annealing temperatures, the average crystallite size diverges between 4.9 and 42.4 nm. The UV–vis absorbance spectra confirmed that nickel ferrites are direct bandgap materials with bandgap ranges from 3.12 to 3.02 eV. The effective mass of the exciton, the exciton Bohr radius, and the dielectric constants of NiFe2O4 nanoparticles were calculated using UV–vis spectroscopy. FTIR analysis displays the existence of two vibrational bonds between 424 and 600 cm−1, corresponding to metal–oxygen interaction at tetrahedral and octahedral sites, respectively. Transmission electron microscope analysis validated the establishment of single-phase nickel ferrite nanoparticles in the range of 2.8–44.7 nm. Nearly zero coercivity and remanence confirmed the superparamagnetic property of the samples. The VSM analysis established the rise in saturation magnetization from 1.65 to 34.67 emu/g and the decrement in coercivity between 0.00481 and 0.00078 T as the annealing temperature increased. The superparamagnetism of the samples is supported by the EPR spectra. The cyclic voltammetry study infers that smaller size NiFe2O4 nanoparticles offer better supercapacitance performance.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


