Isomerization of Hexane and Heptane Mixtures in the Presence of Platinum-Containing Tungstated Zirconia Catalysts
IF 1.3
Q4 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
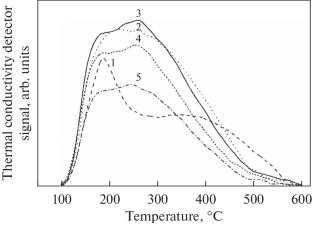
The isomerization of heptane and hexane and their mixtures in the presence of Pt/WO3–ZrO2 catalysts with a tungsten oxide content of 10–35 wt % has been studied. It has been shown that the best parameters of the isomerization of a mixture of C6–C7 alkanes are achieved in the presence of catalysts containing 15–20 wt % WO3. According to temperature-programmed desorption of ammonia, this range of tungsten oxide content is characterized by an increase in the number of acid sites, which, according to IR spectroscopy of adsorbed CO molecules, occurs mostly due to an increase in the number of Brønsted acid sites.
含铂钨氧化锆催化剂存在下正己烷和庚烷混合物的异构化
研究了在氧化钨含量为10 ~ 35wt %的Pt/ WO3-ZrO2催化剂存在下,庚烷和己烷及其混合物的异构化反应。结果表明,C6-C7烷烃混合物异构化的最佳参数是在WO3含量为15-20 wt %的催化剂中实现的。根据氨的程序升温解吸,该范围内氧化钨含量的特征是酸位的增加,根据吸附CO分子的红外光谱,这主要是由于Brønsted酸位的增加。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis in Industry
ENGINEERING, CHEMICAL-
CiteScore
1.30
自引率
14.30%
发文量
21
期刊介绍:
The journal covers the following topical areas:
Analysis of specific industrial catalytic processes: Production and use of catalysts in branches of industry: chemical, petrochemical, oil-refining, pharmaceutical, organic synthesis, fuel-energetic industries, environment protection, biocatalysis; technology of industrial catalytic processes (generalization of practical experience, improvements, and modernization); technology of catalysts production, raw materials and equipment; control of catalysts quality; starting, reduction, passivation, discharge, storage of catalysts; catalytic reactors.Theoretical foundations of industrial catalysis and technologies: Research, studies, and concepts : search for and development of new catalysts and new types of supports, formation of active components, and mechanochemistry in catalysis; comprehensive studies of work-out catalysts and analysis of deactivation mechanisms; studies of the catalytic process at different scale levels (laboratory, pilot plant, industrial); kinetics of industrial and newly developed catalytic processes and development of kinetic models; nonlinear dynamics and nonlinear phenomena in catalysis: multiplicity of stationary states, stepwise changes in regimes, etc. Advances in catalysis: Catalysis and gas chemistry; catalysis and new energy technologies; biocatalysis; nanocatalysis; catalysis and new construction materials.History of the development of industrial catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


