Access and use of immunoglobulins in secondary supportive cancer care: A systematic literature review.
IF 1.5
The journal of medicine access
Pub Date : 2023-10-14
eCollection Date: 2023-01-01
DOI:10.1177/27550834231197315
引用次数: 0
Abstract
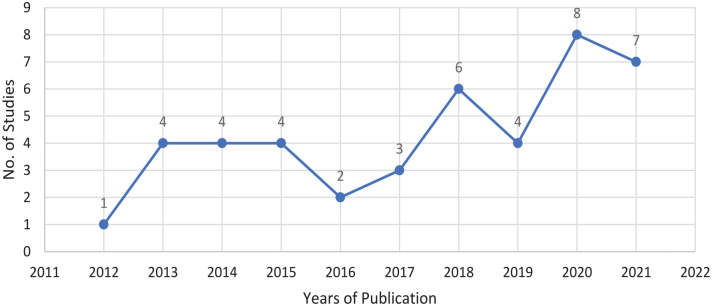
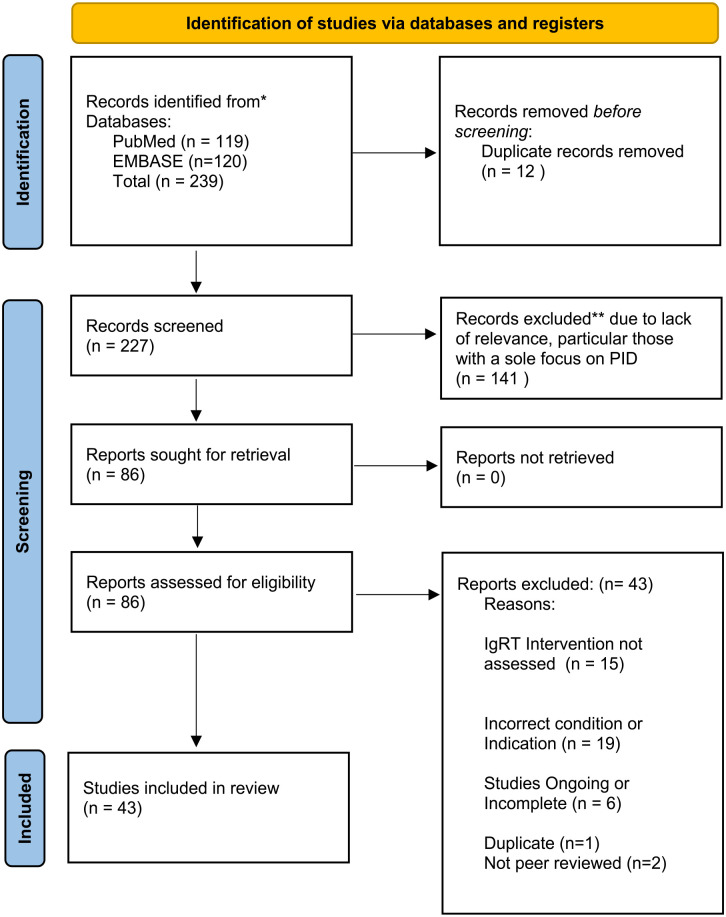
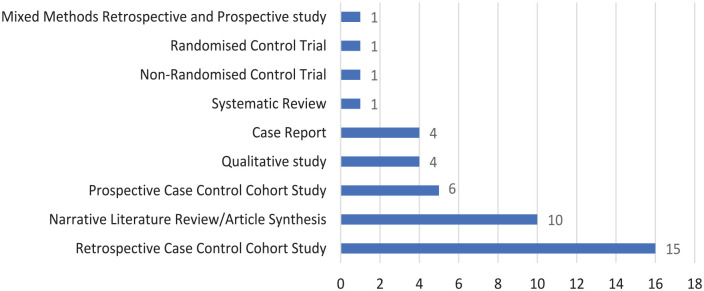
Background: Immunoglobulin replacement therapy (IgRT) benefits patients with primary immuno deficiency (PID) originating from the innate or polygenic defects in the immune system. However, evidence supporting their therapeutic role is not as explicit in secondary immuno deficiency (SID) resulting from the treatment of haematological malignancies. Objectives: This study aimed to (1) create a dataset of relevant research papers, which explore the use of IgRT in SID for analysis, (2) assess the risk of bias within this dataset and (3) study the characteristics of these papers. Design: This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. In addition to the risk of bias, the study characteristics explored in this article included study design, study geographical location and year of publication. Data Sources and Methods: To identify studies relevant to the research question, EMBASE and PubMed databases were searched. The Population, Intervention, Comparison and Outcome (PICO) framework was used to assess study quality. Risk of bias and quality of studies were assessed in accordance with the study design. As one model was not appropriate to assess bias in all articles, several tools were used. Results: A total of 43 studies were identified from the literature search as relevant to the research objective. The most common study design was a retrospective case–control cohort study (n = 16/43), and randomised trials were among the least commonly used approaches (n = 1). Research in this area is occurring around the globe including the United States (n = 7), Italy (n = 7), China, India, Japan and throughout Europe. The annual number of papers in this area has varied from 2012 (n = 1) to 2021 (n = 7). The studies in this article demonstrated a varied risk of bias, with 9 of the 20 cohort studies scoring less than 5 out of 9 stars. Conclusions: Randomised controlled trials are less frequently used to assess access and use of immunoglobulins. More commonly, a retrospective case–control cohort study was used which correlates with the higher risk of bias seen in the studies in this article. Most of the research concerning immunoglobulin use and access occurs in higher-income countries.
免疫球蛋白在二级支持性癌症治疗中的获取和使用:系统文献综述。
背景:免疫球蛋白替代疗法(IgRT)有利于原发性免疫缺陷(PID)患者,该病源于免疫系统的先天或多基因缺陷。然而,支持其治疗作用的证据在血液系统恶性肿瘤治疗引起的继发性免疫缺陷(SID)中并不明确。目的:本研究旨在(1)创建一个相关研究论文的数据集,探索IgRT在SID中的应用进行分析,(2)评估该数据集中的偏倚风险,以及(3)研究这些论文的特征。设计:根据系统评价和荟萃分析(PRISMA)的首选报告项目进行系统评价。除了偏倚的风险外,本文探讨的研究特征还包括研究设计、研究地理位置和发表年份。数据来源和方法:为了确定与研究问题相关的研究,检索了EMBASE和PubMed数据库。使用人口、干预、比较和结果(PICO)框架来评估研究质量。根据研究设计评估偏倚风险和研究质量。由于一个模型不适合评估所有文章中的偏见,因此使用了几种工具。结果:从文献检索中,共有43项研究与研究目标相关。最常见的研究设计是回顾性病例对照队列研究(n = 16/43),随机试验是最不常用的方法之一(n = 1) 。这一领域的研究正在全球范围内进行,包括美国(n = 7) ,意大利(n = 7) ,中国,印度,日本和整个欧洲。该领域的年度论文数量从2012年(n = 1) 至2021(n = 7) 。本文中的研究证明了不同的偏倚风险,20项队列研究中有9项的得分低于9颗星中的5颗星。结论:随机对照试验较少用于评估免疫球蛋白的获取和使用情况。更常见的是,使用了一项回顾性病例对照队列研究,该研究与本文研究中发现的更高的偏倚风险相关。大多数关于免疫球蛋白使用和获取的研究都发生在高收入国家。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


