Phosphine-catalyzed asymmetric aza-Morita–Baylis–Hillman reaction of endocyclic ketimines and activated alkenes†‡
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel phosphine-catalyzed asymmetric aza-Morita–Baylis–Hillman reaction of endocyclic ketimines with vinyl ketone or acrolein has been presented. This approach resulted in densely functionalized adducts with moderate to high yields and enantioselectivities (up to 97% yield, up to 97% ee), with the feature of a C2 tetra-substituted chiral center on a 3-oxindole scaffold. A wide variety of substrates were compatible with this methodology, and diverse enantiomeric products could be obtained through a catalyst switching strategy. A series of transformations further demonstrated the utility of this methodology.
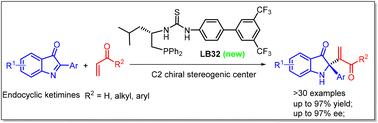
磷化氢催化内环酮亚胺和活性烯烃的不对称aza Morita–Baylis–Hillman反应††
提出了一种新的膦催化的内环酮亚胺与乙烯基酮或丙烯醛的不对称aza Morita–Baylis–Hillman反应。该方法产生了具有中高产率和对映选择性(高达97%产率,高达97%ee)的致密官能化加合物,其特征是在3-羟基吲哚支架上具有C2四取代的手性中心。多种底物与该方法兼容,并且可以通过催化剂切换策略获得不同的对映体产物。一系列的转换进一步证明了这种方法的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


