Demonstrating Partitioning Using a Solvatochromic Dye
IF 2.5
3区 教育学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The partitioning of a dye, Brooker’s merocyanine (MOED), between water and 1-octanol and between water and dichloromethane is strikingly visible because of the dye’s strong solvatochromism, which makes the colors of the two layers different. The color change makes it easy to see that the dye has moved from the organic layer to the water layer or vice versa. A simple comparison of the color of the organic layer to reference solutions makes it possible to estimate rough values of KD, the distribution equilibrium constant.
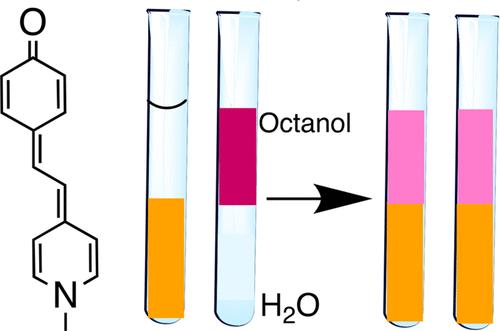
使用溶剂变色染料演示分区
布鲁克亚花青(MOED)染料在水和1-辛醇之间以及在水和二氯甲烷之间的分配非常明显,因为该染料具有强烈的溶剂化色度,这使得两层的颜色不同。颜色的变化很容易看出染料已经从有机层转移到水层,反之亦然。有机层的颜色与参考溶液的简单比较可以估计分布平衡常数KD的粗略值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Education
化学-化学综合
CiteScore
5.60
自引率
50.00%
发文量
465
审稿时长
6.5 months
期刊介绍:
The Journal of Chemical Education is the official journal of the Division of Chemical Education of the American Chemical Society, co-published with the American Chemical Society Publications Division. Launched in 1924, the Journal of Chemical Education is the world’s premier chemical education journal. The Journal publishes peer-reviewed articles and related information as a resource to those in the field of chemical education and to those institutions that serve them. JCE typically addresses chemical content, activities, laboratory experiments, instructional methods, and pedagogies. The Journal serves as a means of communication among people across the world who are interested in the teaching and learning of chemistry. This includes instructors of chemistry from middle school through graduate school, professional staff who support these teaching activities, as well as some scientists in commerce, industry, and government.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


