Efficacy of finerenone in patients with type 2 diabetes, chronic kidney disease and altered markers of liver steatosis and fibrosis: A FIDELITY subgroup analysis
Abstract
Aim
Investigating the effect of finerenone on liver function, cardiovascular and kidney composite outcomes in patients with chronic kidney disease and type 2 diabetes, stratified by their risk of liver steatosis, inflammation and fibrosis.
Materials and Methods
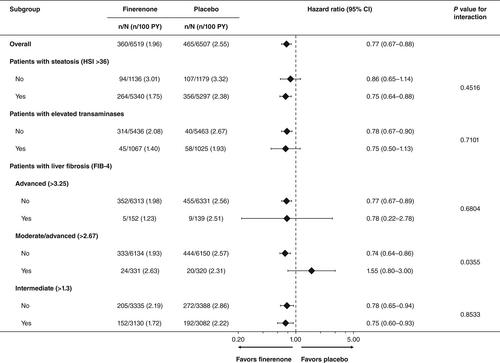
Post hoc analysis stratified patients (N = 13 026) by liver fibrosis and enzymes: high risk of steatosis (hepatic steatosis index >36); elevated transaminases [alanine transaminase (ALT) >33 (males) and >25 IU/L (females)]; and fibrosis-4 (FIB-4) index scores >3.25, >2.67 and >1.30. Liver enzymes were assessed by changes in ALT, aspartate aminotransferase and gamma-glutamyl transferase. Composite kidney outcome was defined as onset of kidney failure, sustained estimated glomerular filtration rate decline ≥57% from baseline over ≥4 weeks or kidney death. Composite cardiovascular outcome was defined as cardiovascular death, non-fatal myocardial infarction, non-fatal stroke or hospitalization for heart failure.
Results
ALT, aspartate aminotransferase and gamma-glutamyl transferase levels were consistent between treatment groups and remained stable throughout. Finerenone consistently reduced the risk of composite kidney outcome, irrespective of altered liver tests. Higher FIB-4 score was associated with higher incidence rates of composite cardiovascular outcome. Finerenone reduced the risk of composite cardiovascular outcome versus placebo in FIB-4 subgroups by 52% (>3.25), 39% (>2.67) and 24% (>1.30) (p values for interaction = .01, .13 and .03, respectively).
Conclusions
Finerenone has neutral effects on liver parameters in patients with chronic kidney disease and type 2 diabetes. Finerenone showed robust and consistent kidney benefits in patients with altered liver tests, and profound cardiovascular benefits even in patients with higher FIB-4 scores who were at high risk of developing cardiovascular complications.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


