Post COVID-19 burden: focus on the short-term condition.
IF 4.3
Acupuncture and herbal medicine
Pub Date : 2022-09-01
Epub Date: 2022-12-08
DOI:10.1097/HM9.0000000000000036
引用次数: 2
Abstract
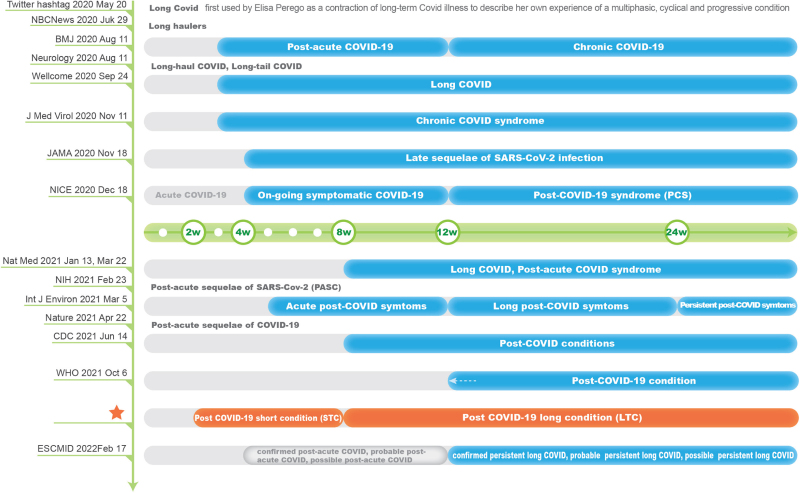
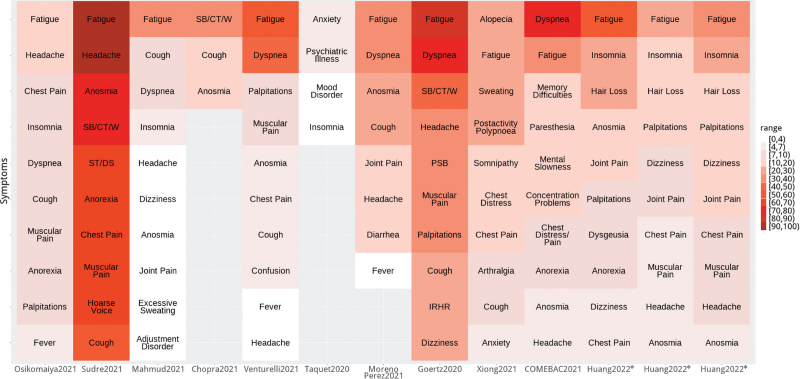
The catastrophic pandemic of the coronavirus disease 2019 (COVID-19) has caused serious harm to human life and global social economy. As of June 13, 2022, there were more than 530 million confirmed cases of COVID19 and over 6.3 million deaths[1]. During the past 2 years, global studies on prevention and therapies for COVID19 have achieved a series of promising findings, including antiviral drugs (Remdesivir, Molnupiravir, Paxlovid) and monoclonal antibodies (Bamlanivimab and Etesevimab, Sotrovimab, Casirivimab and Imdevimab)[2]. World Health Organization (WHO) has validated 11 vaccines for the emergency use listing (EUL)[3]. As of June 13, 2022, more than 11.9 billion vaccine doses have been administered worldwide[1]. In China, integrated using of traditional Chinese medicine (TCM) and Western medicine is one of the highlights in fighting COVID-19. “Three formulations and three drugs” (Jinhua Qinggan granules, Lianhua Qingwen capsule, Xuebijing injection, and Qingfei Paidu decoction, Huashi Baidu decoction, Xuanfei Baidu decoction) have been approved by National Medical Products Administration (NMPA) as new drugs or adding indications for COVID-19[4]. The new drugs and vaccines had become effective weapons in the fight against the pandemic.
新冠肺炎后的负担:关注短期状况。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


