Single-cell lineage capture across genomic modalities with CellTag-multi reveals fate-specific gene regulatory changes
IF 33.1
1区 生物学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Complex gene regulatory mechanisms underlie differentiation and reprogramming. Contemporary single-cell lineage-tracing (scLT) methods use expressed, heritable DNA barcodes to combine cell lineage readout with single-cell transcriptomics. However, reliance on transcriptional profiling limits adaptation to other single-cell assays. With CellTag-multi, we present an approach that enables direct capture of heritable random barcodes expressed as polyadenylated transcripts, in both single-cell RNA sequencing and single-cell Assay for Transposase Accessible Chromatin using sequencing assays, allowing for independent clonal tracking of transcriptional and epigenomic cell states. We validate CellTag-multi to characterize progenitor cell lineage priming during mouse hematopoiesis. Additionally, in direct reprogramming of fibroblasts to endoderm progenitors, we identify core regulatory programs underlying on-target and off-target fates. Furthermore, we reveal the transcription factor Zfp281 as a regulator of reprogramming outcome, biasing cells toward an off-target mesenchymal fate. Our results establish CellTag-multi as a lineage-tracing method compatible with multiple single-cell modalities and demonstrate its utility in revealing fate-specifying gene regulatory changes across diverse paradigms of differentiation and reprogramming. Lineage tracing using both transcriptomics and chromatin accessibility provides mechanistic insights into cell fate.
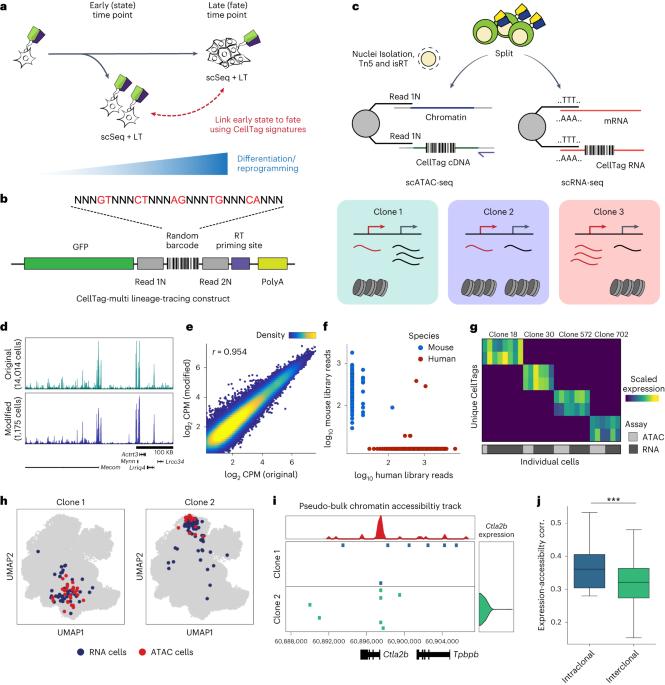
CellTag multi跨基因组模式捕获单细胞谱系揭示了命运特异性基因调控变化。
复杂的基因调控机制是分化和重编程的基础。当代的单细胞谱系追踪(scLT)方法使用表达的、可遗传的DNA条形码将细胞谱系读数与单细胞转录组学相结合。然而,对转录谱的依赖限制了对其他单细胞测定的适应。利用CellTag multi,我们提出了一种方法,可以在单细胞RNA测序和使用测序分析的转座酶可及染色质单细胞分析中直接捕获以聚腺苷酸转录物形式表达的可遗传随机条形码,从而实现转录和表观基因组细胞状态的独立克隆跟踪。我们验证CellTag multi在小鼠造血过程中表征祖细胞谱系启动。此外,在成纤维细胞直接重编程为内胚层祖细胞的过程中,我们确定了靶向和脱靶命运的核心调控程序。此外,我们揭示了转录因子Zfp281作为重编程结果的调节因子,使细胞偏离靶向间充质命运。我们的研究结果将CellTag multi确立为一种与多种单细胞模式兼容的谱系追踪方法,并证明了它在揭示分化和重编程的不同范式中的命运指定基因调控变化方面的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature biotechnology
工程技术-生物工程与应用微生物
CiteScore
63.00
自引率
1.70%
发文量
382
审稿时长
3 months
期刊介绍:
Nature Biotechnology is a monthly journal that focuses on the science and business of biotechnology. It covers a wide range of topics including technology/methodology advancements in the biological, biomedical, agricultural, and environmental sciences. The journal also explores the commercial, political, ethical, legal, and societal aspects of this research.
The journal serves researchers by providing peer-reviewed research papers in the field of biotechnology. It also serves the business community by delivering news about research developments. This approach ensures that both the scientific and business communities are well-informed and able to stay up-to-date on the latest advancements and opportunities in the field.
Some key areas of interest in which the journal actively seeks research papers include molecular engineering of nucleic acids and proteins, molecular therapy, large-scale biology, computational biology, regenerative medicine, imaging technology, analytical biotechnology, applied immunology, food and agricultural biotechnology, and environmental biotechnology.
In summary, Nature Biotechnology is a comprehensive journal that covers both the scientific and business aspects of biotechnology. It strives to provide researchers with valuable research papers and news while also delivering important scientific advancements to the business community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


