Metal-free C(sp3)-H Bromination: Synthesis of Phenacyl bromide and Benzyl bromide derivatives
IF 2
4区 化学
Q3 Chemistry
引用次数: 0
Abstract
A metal-free C(sp3)-H bromination methodology has been developed for aromatic ketones and some substituted toluenes under ambient conditions. The reaction for the toluenes is mediated by PhI(OAc)2 (iodobenzene diacetate) in presence of KBr, while the bromination in aromatic ketones requires additional assistance of p-TsOH.H2O and BF3.Et2O.
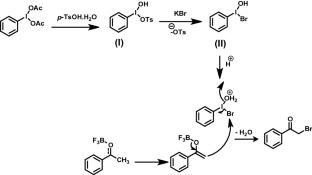
Graphical abstract
Synopsis: Synthesis of phenacyl bromides has been achieved under transition metal-free conditions. Some observations on substituted toluene systems have also been reported. In both cases, C(sp3)-H bromination has taken place at room temperature in the presence of phenyliodine diacetate (PIDA).
无金属C(sp3)-H溴化:苯那基溴和苯溴衍生物的合成
本文建立了一种无金属的C(sp3)-H溴化方法,用于芳香酮和某些取代甲苯的环境反应。甲苯的反应是在KBr存在下由PhI(OAc)2(二乙酸碘苯)介导的,而芳香酮的溴化则需要对tsoh的辅助。H2O和bf3, et2o。摘要:在无过渡金属的条件下合成了苯那基溴化物。还报道了对取代甲苯体系的一些观察结果。在这两种情况下,C(sp3)-H溴化都是在室温下,在二乙酸苯碘(PIDA)的存在下发生的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Sciences
Chemistry-General Chemistry
CiteScore
2.90
自引率
5.90%
发文量
107
审稿时长
12 months
期刊介绍:
Journal of Chemical Sciences is a monthly journal published by the Indian Academy of Sciences. It formed part of the original Proceedings of the Indian Academy of Sciences – Part A, started by the Nobel Laureate Prof C V Raman in 1934, that was split in 1978 into three separate journals. It was renamed as Journal of Chemical Sciences in 2004. The journal publishes original research articles and rapid communications, covering all areas of chemical sciences. A significant feature of the journal is its special issues, brought out from time to time, devoted to conference symposia/proceedings in frontier areas of the subject, held not only in India but also in other countries.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


