Thiostrepton alleviates experimental colitis by promoting RORγt ubiquitination and modulating dysbiosis
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
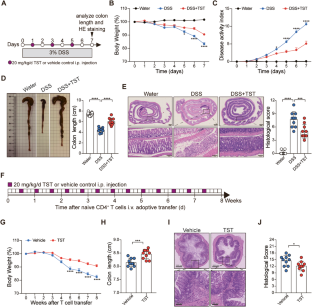
Thiostrepton (TST) is a natural antibiotic with pleiotropic properties. This study aimed to elucidate the therapeutic effect of TST on experimental colitis and identify its targets. The effect of TST on colon inflammation was evaluated in a dextran sulfate sodium (DSS)-induced colitis model and a T-cell transfer colitis model. The therapeutic targets of TST were investigated by cytokine profiling, immunophenotyping and biochemical approaches. The effect of TST on the gut microbiota and its contribution to colitis were evaluated in mice with DSS-induced colitis that were subjected to gut microbiota depletion and fecal microbiota transplantation (FMT). Alterations in the gut microbiota caused by TST were determined by 16S rDNA and metagenomic sequencing. Here, we showed that TST treatment significantly ameliorated colitis in the DSS-induced and T-cell transfer models. Specifically, TST targeted the retinoic acid-related orphan nuclear receptor RORγt to reduce the production of IL-17A by γδ T cells, type 3 innate lymphoid cells (ILC3s) and Th17 cells in mice with DSS-induced colitis. Similarly, TST selectively prevented the development of Th17 cells in the T-cell transfer colitis model and the differentiation of naïve CD4+ T cells into Th17 cells in vitro. Mechanistically, TST induced the ubiquitination and degradation of RORγt by promoting the binding of Itch to RORγt. Moreover, TST also reversed dysbiosis to control colonic inflammation. Taken together, these results from our study describe the previously unexplored role of TST in alleviating colonic inflammation by reducing IL-17A production and modulating dysbiosis, suggesting that TST is a promising candidate drug for the treatment of IBD.
硫链球菌素通过促进RORγt泛素化和调节微生态失调来减轻实验性结肠炎。
硫链菌素(TST)是一种具有多效性的天然抗生素。本研究旨在阐明TST对实验性结肠炎的治疗作用并确定其靶点。在右旋糖酐硫酸钠(DSS)诱导的结肠炎模型和T细胞转移性结肠炎模型中评估TST对结肠炎症的影响。通过细胞因子谱、免疫表型和生化方法研究TST的治疗靶点。在经历肠道微生物群耗竭和粪便微生物群移植(FMT)的DSS诱导的结肠炎小鼠中评估TST对肠道微生物群的影响及其对结肠炎的贡献。通过16S rDNA和宏基因组测序确定TST引起的肠道微生物群的改变。在这里,我们发现TST治疗在DSS诱导的和T细胞转移模型中显著改善了结肠炎。具体而言,TST靶向视黄酸相关孤儿核受体RORγt,以减少DSS诱导的结肠炎小鼠中γδt细胞、3型固有淋巴细胞(ILC3s)和Th17细胞产生IL-17A。类似地,TST在T细胞转移性结肠炎模型中选择性地阻止Th17细胞的发育,并在体外阻止幼稚的CD4+T细胞分化为Th17细胞。从机制上讲,TST通过促进瘙痒与RORγt的结合,诱导RORγt的泛素化和降解。此外,TST还可以逆转微生态失调以控制结肠炎症。总之,我们研究的这些结果描述了TST通过减少IL-17A的产生和调节微生态失调在减轻结肠炎症中的作用,这表明TST是治疗IBD的一种有前途的候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.
文献相关原料
| 公司名称 | 产品信息 | 采购帮参考价格 |
|---|

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


