Diabetes remission and relapse following an intensive metabolic intervention combining insulin glargine/lixisenatide, metformin and lifestyle approaches: Results of a randomised controlled trial
Abstract
Aim
Non-surgical options for inducing type 2 diabetes remission are limited. We examined whether remission can be achieved by combining lifestyle approaches and short-term intensive glucose-lowering therapy.
Methods
In this trial, 160 patients with type 2 diabetes on none to two diabetes medications other than insulin were randomised to (a) an intervention comprising lifestyle approaches, insulin glargine/lixisenatide and metformin, or (b) standard care. Participants with glycated haemoglobin (HbA1c) <7.3% (56 mmol/mol) at 12 weeks were asked to stop diabetes medications and were followed for an additional 52 weeks. The primary outcome was diabetes relapse defined as HbA1c ≥6.5% (48 mmol/mol) at 24 weeks or thereafter, capillary glucose ≥10 mmol/L on ≥50% of readings, or use of diabetes medications, analysed as time-to-event. Main secondary outcomes included complete or partial diabetes remission at 24, 36, 48 and 64 weeks defined as HbA1c <6.5% (48 mmol/mol) off diabetes medications since 12 weeks after randomisation. A hierarchical testing strategy was applied.
Results
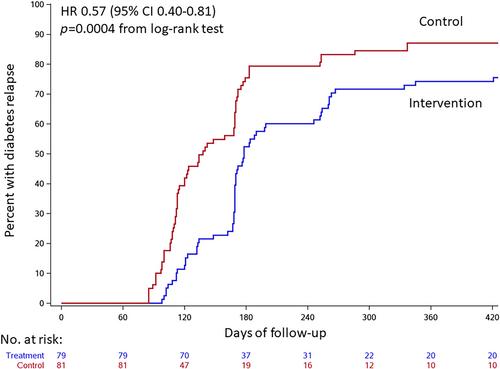
The intervention significantly reduced the hazard of diabetes relapse by 43% (adjusted hazard ratio 0.57, 95% confidence interval 0.40-0.81; p = .002). Complete or partial diabetes remission was achieved in 30 (38.0%) intervention group participants versus 16 (19.8%) controls at 24 weeks and 25 (31.6%) versus 14 (17.3%) at 36 weeks [relative risk 1.92 (95% confidence interval 1.14-3.24) and 1.83 (1.03-3.26), respectively]. The relative risk of diabetes remission in the intervention versus control group was 1.88 (1.00-3.53) at 48 weeks and 2.05 (0.98-4.29) at 64 weeks.
Conclusions
A 12-week intensive intervention comprising insulin glargine/lixisenatide, metformin and lifestyle approaches can induce remission of diabetes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


