A 1-year pilot study of intralymphatic injections of GAD-alum in individuals with latent autoimmune diabetes in adults (LADA) with signs of high immunity: No safety concerns and resemblance to juvenile type 1 diabetes
Abstract
Aims
To test, for the first time in latent autoimmune diabetes in adults (LADA), the effects of autoantigen-specific immunotherapy by intralymphatic administration of aluminium-formulated recombinant human glutamic acid decarboxylase 65 (GAD-alum); specifically, to test if this treatment is safe, to test whether it induces a strong immunological response akin to a similar protocol in type 1 diabetes and to look for associations with preserved beta-cell function.
Materials and Methods
Three GAD-alum injections, 4 μg each, were administered 1 month apart into an inguinal lymph node in 14 people with newly diagnosed LADA (age 30-62 years) presenting with high levels of antibodies against glutamic acid decarboxylase (GADA). Adverse effects, immunological variables and beta-cell function were monitored, with detailed measurements at 5 and 12 months from baseline.
Results
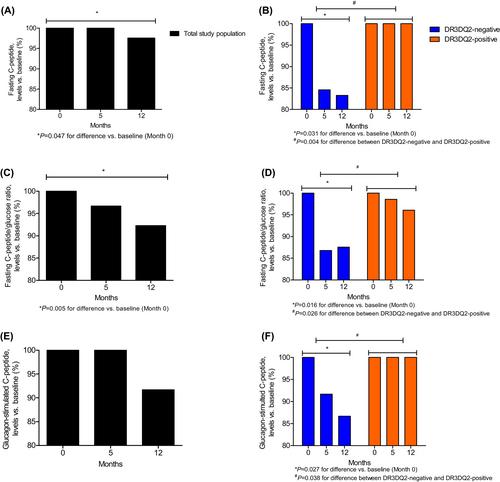
Clinical adverse effects were minor and transient and measured laboratory variables were unaffected. All participants completed the study. Treatment raised levels of GADA, elicited strong effects on reactivity of peripheral blood mononuclear cells to GAD and raised cytokine/chemokine levels. Beta-cell function appeared stable preferentially in the seven participants carrying human leukocyte antigen (HLA) haplotypes DR3DQ2, as assessed by C-peptide glucagon tests (P < 0.05 vs. seven non-carriers).
Conclusion
Intralymphatic treatment with GAD-alum in LADA is without clinical or other safety concerns over a 12-month period. As in a similar protocol used in type 1 diabetes, treatment exerts a strong immunological impact and is compatible with protection of beta-cell function preferentially in HLA-DR3DQ2 LADA patients. These findings pave the way for a randomized controlled trial in this important subgroup of LADA patients.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


