Interaction of 2-Amino-1,3,4-thiadiazoles with 1,2,4-Triazine-5-carbonitriles
IF 1
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
A possibility of preparation of 1,2,4-triazines with a residue of 2-amino-1,3,4-thiadiazoles at the C5 position by the solvent-free reaction of ipso-amination of 3,6-di(het)aryl-1,2,4-triazine-5-carbonitriles has been studied. It has been found that the reaction also leads to the corresponding 5-amino-1,2,4-triazines as minor products.
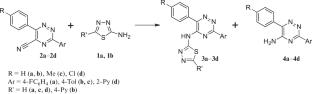
2-氨基-1,3,4-噻二唑与1,2,4-三嗪-5-碳腈的相互作用
研究了以2-氨基-1,3,4-噻二唑为C5位残基,通过3,6-二(二)芳基-1,2,4-三嗪-5-碳腈的异胺化反应制备1,2,4-三嗪的可能性。该反应还可生成相应的5-氨基-1,2,4-三嗪作为次要产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Doklady Chemistry
化学-化学综合
CiteScore
1.20
自引率
12.50%
发文量
7
审稿时长
6-12 weeks
期刊介绍:
Doklady Chemistry is a journal that publishes new research in chemistry and chemical engineering of great significance. Initially the journal was a forum of the Russian Academy of Science and published only best contributions from Russia in the form of short articles. Now the journal welcomes submissions from any country in the English or Russian language. Every manuscript must be recommended by Russian or foreign members of the Russian Academy of Sciences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


