First Therapeutic Approval for Eosinophilic Esophagitis.
IF 0.7
Q3 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 9
Abstract
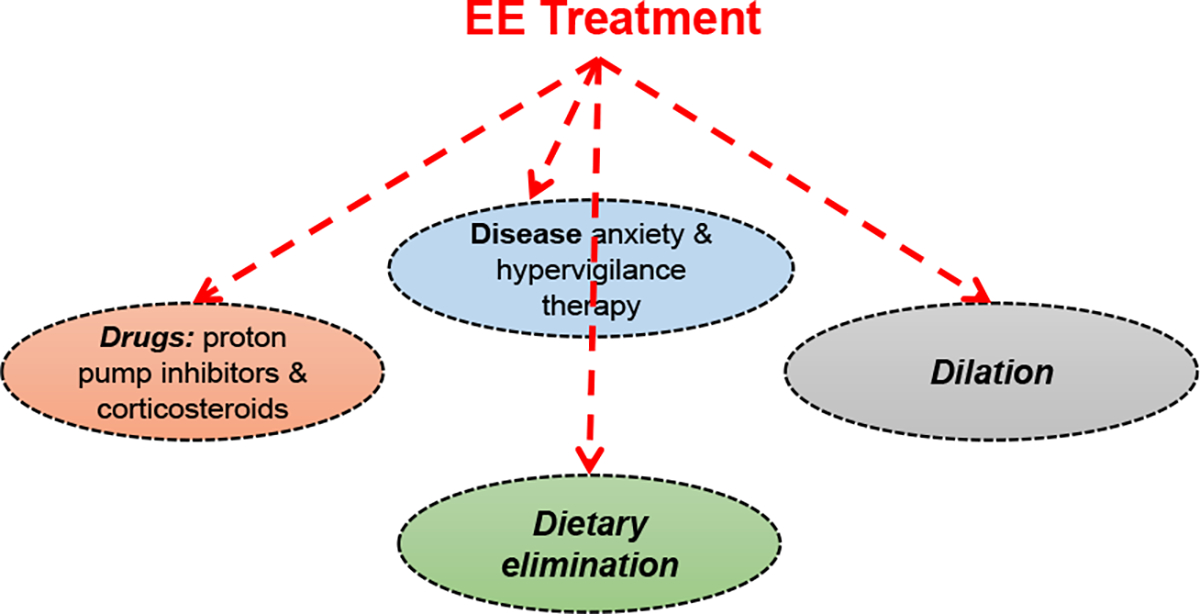
Eosinophilic esophagitis (EE) is a chronic, immune-mediated or antigen-mediated esophageal disease. Treatment for patients with EE can be challenging with no previously approved medications. Current management strategies follow the four D's paradigm of drugs, dietary elimination, dilation, and disease anxiety and hypervigilance therapy. On 20 May 2022, dupilumab was approved by FDA for EE. A dose of 300 mg dupilumab weekly significantly improved signs and symptoms of EE compared to placebo in a phase 3 trial. The approval of dupilumab will fulfill an unmet need for the increasing number of patients with EE.
嗜酸性粒细胞性食管炎首次获得治疗批准。
嗜酸性粒细胞性食管炎(EE)是一种慢性、免疫介导或抗原介导的食管疾病。由于没有先前批准的药物,情感表达患者的治疗可能具有挑战性。目前的管理策略遵循四个D的范例:药物、饮食消除、扩张、疾病焦虑和过度警惕治疗。2022年5月20日,dupilumab被FDA批准用于EE。在一项3期试验中,与安慰剂相比,每周剂量300 mg dupilumab可显著改善EE的体征和症状。dupilumab的批准将满足越来越多的EE患者的需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Gastroenterology Insights
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
2.80
自引率
3.40%
发文量
35
审稿时长
10 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


