EGFR T790M Mutation Detection in Patients With Non-Small Cell Lung Cancer After First Line EGFR TKI Therapy: Summary of Results in a Three-Year Period and a Comparison of Commercially Available Detection Kits.
Pathology oncology research : POR
Pub Date : 2022-10-05
eCollection Date: 2022-01-01
DOI:10.3389/pore.2022.1610607
引用次数: 2
Abstract
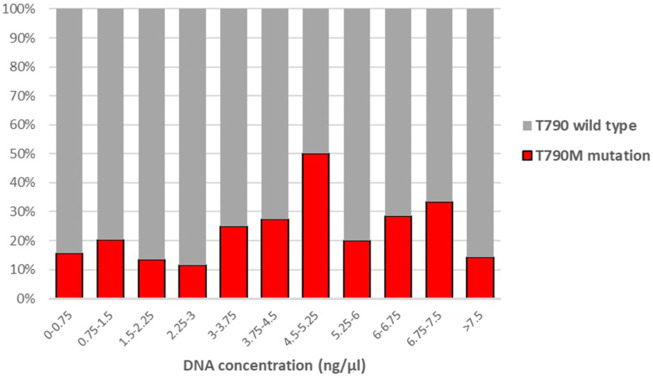
EGFR mutation in non-small cell lung cancer (NSCLC) offers a potential therapeutic target for tyrosine kinase inhibitor (TKI) therapy. The majority of these cases, however eventually develop therapy resistance, mainly by acquiring EGFR T790M mutation. Recently, third-generation TKIs have been introduced to overcome T790M mutation-related resistance. Cell free circulating tumor DNA (liquid biopsy) has emerged as a valuable alternative method for T790M mutation detection during patient follow up, when a tissue biopsy cannot be obtained for analysis. In this study, we summarized our experience with Super-ARMS EGFR Mutation Detection Kit (AmoyDx) on 401 samples of 242 NSCLC patients in a 3-year period in Hungary, comprising 364 plasma and 37 non-plasma samples. We also compared the performance of two commercially available detection kits, the cobas EGFR Mutation test v2 (Roche) and the Super-ARMS EGFR Mutation Detection Kit (AmoyDx). The same activating EGFR mutation was detected with the AmoyDx kit as in the primary tumor in 45.6% of the samples. T790M mutation was identified in 48.1% of the samples containing activating EGFR mutation. The detection rate of T790M mutation was not dependent on the DNA concentration of the plasma sample and there was no considerable improvement in mutation detection rate after a second, subsequent plasma sample. The concordance of EGFR activating mutation detection was 89% between the two methods, while this was 93% for T790M mutation detection. The AmoyDx kit, however showed an overall higher detection rate of T790M mutation compared to the cobas kit (p = 0.014). T790M mutation was detected at 29.8% of the patients if only plasma samples were available for analysis, while the detection rate was 70.2% in non-plasma samples. If the activating EGFR was detected in the plasma samples, the detection rate of T790M mutation was 42.4%. Although non-plasma samples provided a superior T790M mutation detection rate, we found that liquid biopsy can offer a valuable tool for T790M mutation detection, when a tissue biopsy is not available. Alternatively, a liquid biopsy can be used as a screening test, when re-biopsy should be considered in case of wild-type results.
一线EGFR TKI治疗后非小细胞肺癌患者的EGFR T790M突变检测:三年的结果总结和市售检测试剂盒的比较
非小细胞肺癌(NSCLC)的EGFR突变为酪氨酸激酶抑制剂(TKI)治疗提供了一个潜在的治疗靶点。然而,这些病例中的大多数最终会产生治疗耐药性,主要是通过获得EGFR T790M突变。最近,第三代tki被引入以克服T790M突变相关的耐药性。当无法获得组织活检进行分析时,游离细胞循环肿瘤DNA(液体活检)已成为患者随访期间检测T790M突变的一种有价值的替代方法。在这项研究中,我们总结了Super-ARMS EGFR突变检测试剂盒(AmoyDx)在匈牙利242例NSCLC患者的401份样本中3年期间的经验,其中包括364份血浆样本和37份非血浆样本。我们还比较了两种市售检测试剂盒的性能,cobas EGFR突变检测试剂盒v2 (Roche)和Super-ARMS EGFR突变检测试剂盒(AmoyDx)。在45.6%的样本中,AmoyDx试剂盒检测到与原发肿瘤相同的激活EGFR突变。在48.1%的EGFR激活突变样本中鉴定出T790M突变。T790M突变的检出率不依赖于血浆样品的DNA浓度,并且在第二次后续血浆样品后,突变检出率没有显着提高。两种方法检测EGFR激活突变的一致性为89%,而T790M突变检测的一致性为93%。然而,与cobas试剂盒相比,AmoyDx试剂盒对T790M突变的总体检出率更高(p = 0.014)。如果只进行血浆样本分析,T790M突变检出率为29.8%,而在非血浆样本中,T790M突变检出率为70.2%。如果在血浆样本中检测到激活EGFR,则T790M突变的检出率为42.4%。虽然非血浆样本提供了更高的T790M突变检出率,但我们发现,当不能进行组织活检时,液体活检可以提供一种有价值的T790M突变检测工具。或者,液体活检可以作为筛选试验,如果出现野生型结果,则应考虑重新活检。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


