{"title":"FRET-FLIM to Determine Protein Interactions and Membrane Topology of Enzyme Complexes.","authors":"Tatiana Spatola Rossi, Charlotte Pain, Stanley W Botchway, Verena Kriechbaumer","doi":"10.1002/cpz1.598","DOIUrl":null,"url":null,"abstract":"<p><p>Determining protein-protein interactions is vital for gaining knowledge on cellular and metabolic processes including enzyme complexes and metabolons. Förster resonance energy transfer with fluorescence lifetime imaging microscopy (FRET-FLIM) is an advanced imaging methodology that allows for the quantitative detection of protein-protein interactions. In this method, proteins of interest for interaction studies are fused to different fluorophores such as enhanced green fluorescent protein (eGFP; donor molecule) and monomeric red fluorescent protein (mRFP; acceptor molecule). Energy transfer between the two fluorophore groups can only occur efficiently when the proteins of interest are in close physical proximity, around ≤10 nm, and therefore are most likely interacting. FRET-FLIM measures the decrease in excited-state lifetime of the donor fluorophore (eGFP) with and without the presence of the acceptor (mRFP) and can therefore give information on protein-protein interactions and the membrane topology of the tested protein. Here we describe the production of fluorescent protein fusions for FRET-FLIM analysis in tobacco leaf epidermal cells using Agrobacterium-mediated plant transformation and a FRET-FLIM data acquisition and analysis protocol in plant cells. These protocols are applicable and can be adapted for both membrane and soluble proteins in different cellular localizations. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: Protein expression in tobacco leaf cells via transient Agrobacterium-mediated plant transformation Basic Protocol 2: FRET-FLIM data acquisition and analysis.</p>","PeriodicalId":11174,"journal":{"name":"Current Protocols","volume":" ","pages":"e598"},"PeriodicalIF":0.0000,"publicationDate":"2022-10-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11648839/pdf/","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current Protocols","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.1002/cpz1.598","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
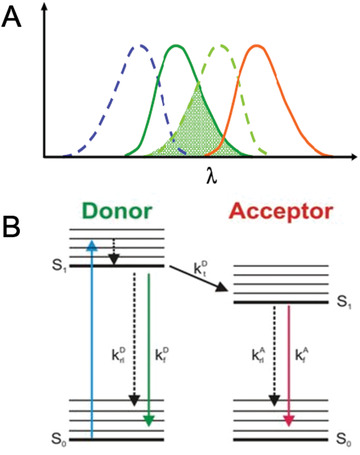
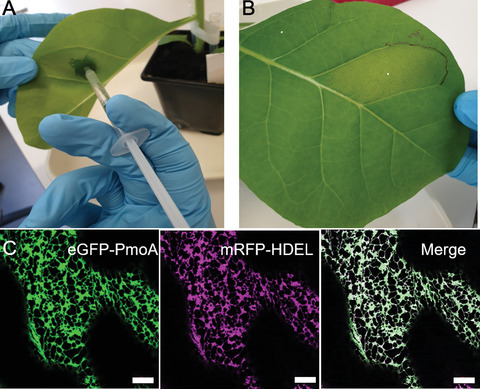
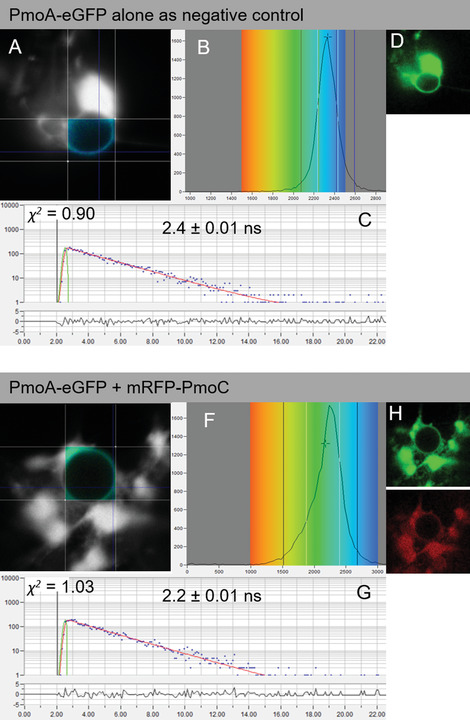
Determining protein-protein interactions is vital for gaining knowledge on cellular and metabolic processes including enzyme complexes and metabolons. Förster resonance energy transfer with fluorescence lifetime imaging microscopy (FRET-FLIM) is an advanced imaging methodology that allows for the quantitative detection of protein-protein interactions. In this method, proteins of interest for interaction studies are fused to different fluorophores such as enhanced green fluorescent protein (eGFP; donor molecule) and monomeric red fluorescent protein (mRFP; acceptor molecule). Energy transfer between the two fluorophore groups can only occur efficiently when the proteins of interest are in close physical proximity, around ≤10 nm, and therefore are most likely interacting. FRET-FLIM measures the decrease in excited-state lifetime of the donor fluorophore (eGFP) with and without the presence of the acceptor (mRFP) and can therefore give information on protein-protein interactions and the membrane topology of the tested protein. Here we describe the production of fluorescent protein fusions for FRET-FLIM analysis in tobacco leaf epidermal cells using Agrobacterium-mediated plant transformation and a FRET-FLIM data acquisition and analysis protocol in plant cells. These protocols are applicable and can be adapted for both membrane and soluble proteins in different cellular localizations. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: Protein expression in tobacco leaf cells via transient Agrobacterium-mediated plant transformation Basic Protocol 2: FRET-FLIM data acquisition and analysis.
FRET-FLIM测定蛋白质相互作用和酶复合物的膜拓扑结构。
确定蛋白质-蛋白质相互作用对于获得细胞和代谢过程(包括酶复合物和代谢)的知识至关重要。Förster共振能量转移与荧光寿命成像显微镜(FRET-FLIM)是一种先进的成像方法,允许定量检测蛋白质-蛋白质相互作用。在这种方法中,对相互作用研究感兴趣的蛋白质被融合到不同的荧光团,如增强绿色荧光蛋白(eGFP;供体分子)和单体红色荧光蛋白(mRFP;受体分子)。两个荧光基团之间的能量转移只有在感兴趣的蛋白质在物理上接近时才能有效地发生,大约在≤10 nm左右,因此最有可能相互作用。FRET-FLIM测量供体荧光团(eGFP)在有和没有受体(mRFP)存在的情况下激发态寿命的减少,因此可以提供蛋白质-蛋白质相互作用和被测蛋白质的膜拓扑结构的信息。在这里,我们描述了利用农杆菌介导的植物转化和植物细胞中FRET-FLIM数据采集和分析方案,在烟草叶表皮细胞中生产用于FRET-FLIM分析的荧光蛋白融合物。这些方案是适用的,可以适用于膜和可溶性蛋白在不同的细胞定位。©2022作者。Wiley期刊有限责任公司发表的当前方案。基本方案1:通过瞬时农杆菌介导的植物转化在烟叶细胞中的蛋白质表达。基本方案2:FRET-FLIM数据采集和分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。




 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


