Commercial Low Molecular Weight Heparins — Patent Ecosystem and Technology Paradigm for Quality Characterization
Abstract
Abstract
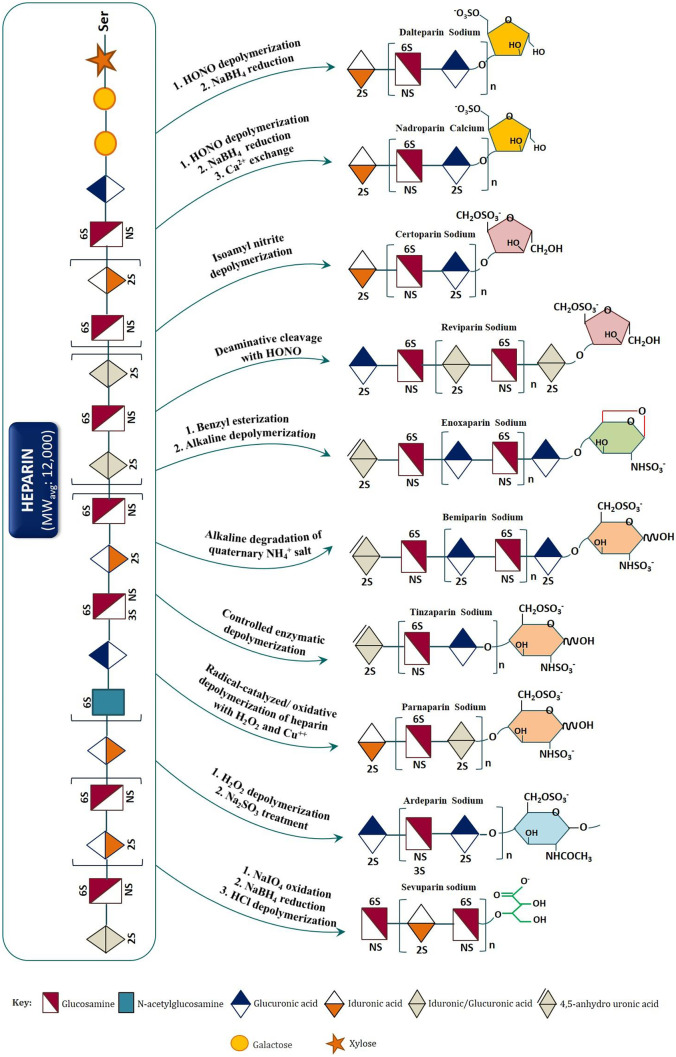
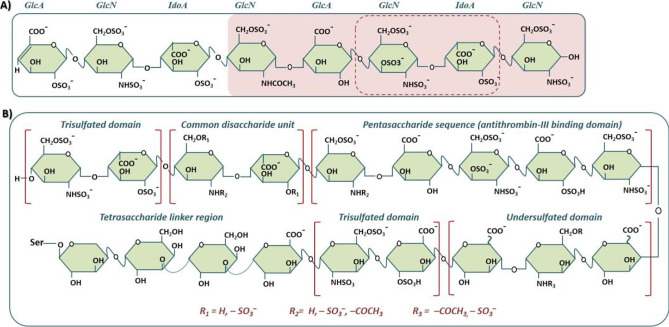
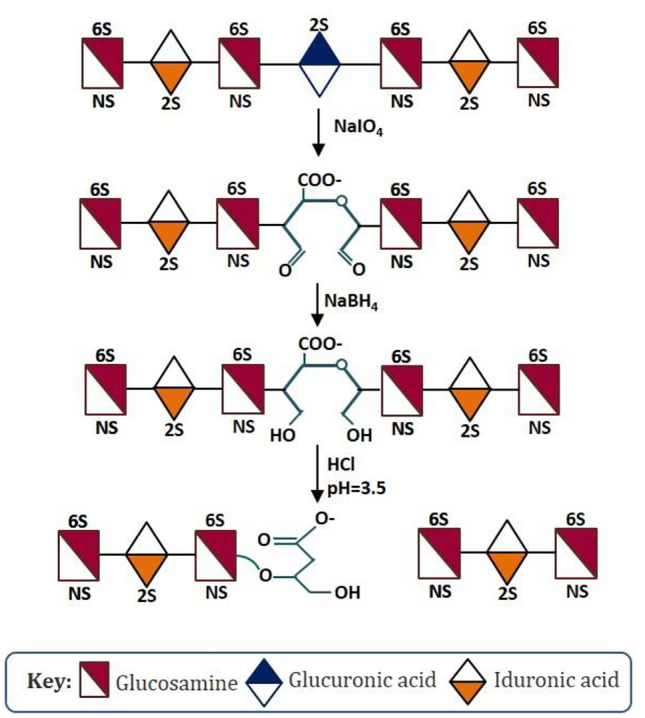
Heparin is a subject of ever-growing interest for laboratory researchers and pharmaceutical industry. One of the driving factors is its critical life-saving drug status, which during the COVID-19 pandemic has assumed a central role in disease treatment and/or prevention. Apart, heparin is one amongst few drugs enjoying a “demand constant” status. In 2020, heparin market size was valued to US$6.5 bn., and given the ongoing stability in the COVID-19 health crisis, it is expected to reach US$11.43 bn. by 2027 with yearly growth rate momentum (CAGR) of 3.9% during the forecast period (Pepi et al., Mol Cell Proteomics 20:100,025, 2021). As patent is a limited monopoly, every year, many patents on low molecular weight heparin (LMWH; a chemically or enzymatically degraded product of unfractionated heparin) are losing market exclusivity worldwide, inviting the generic/biosimilar drug manufacturers to capture market share with cheaper drug products. By tracking patent expiration, drugs in patent litigation, regulatory setbacks for innovator companies (such as those seeking data exclusivity or patent term extension), or other unexpected events affecting market demand and competition, generics can make investment decisions in manufacturing off-patent LMWH drug products of commercial significance. However, given the US Food and Drug Administration (FDA), European Medicine Agency (EMA), Drug Regulatory Authority of Pakistan (DRAP), and other regulatory authorities scientifically rigorous standards for generic/biosimilar LMWH drug products marketing approval, the market is secured and momentous for drug makers that could demonstrate through scientific and clinical dataset that the generic/biosimilar LMWH drug product is of the same quality and purity as the innovator drug product. This study presents an overview of the patent landscape of commercially available LMWHs and advanced analytical techniques for their structural and biochemical characterization for quality control and quality assurance during manufacturing and post-marketing. The study also covers FDA, EMA, Health Canada, and DRAP’s current approaches to evaluating the generic/biosimilar LMWH drug products for quality, safety including immunogenicity, and efficacy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


