Acid–base Homeostasis and Implications to the Phenotypic Behaviors of Cancer
IF 7.9
2区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
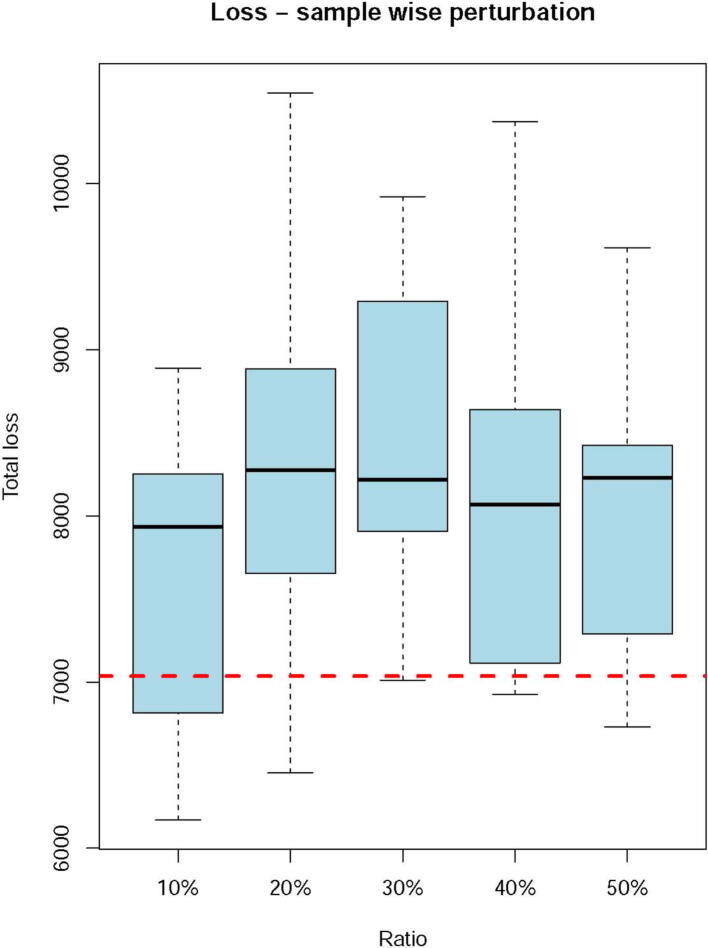
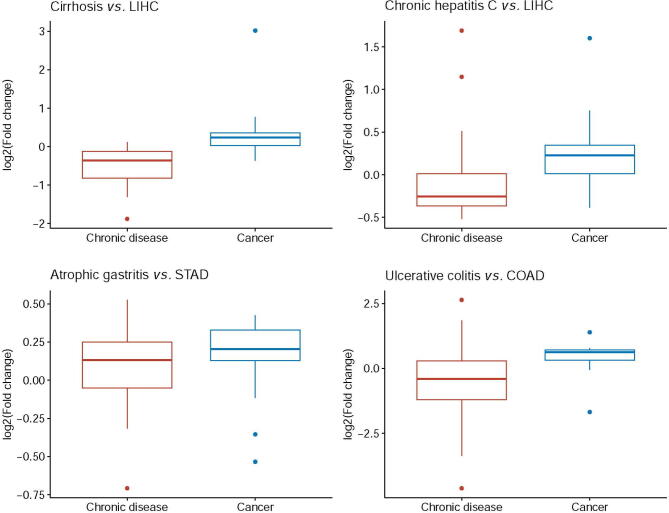
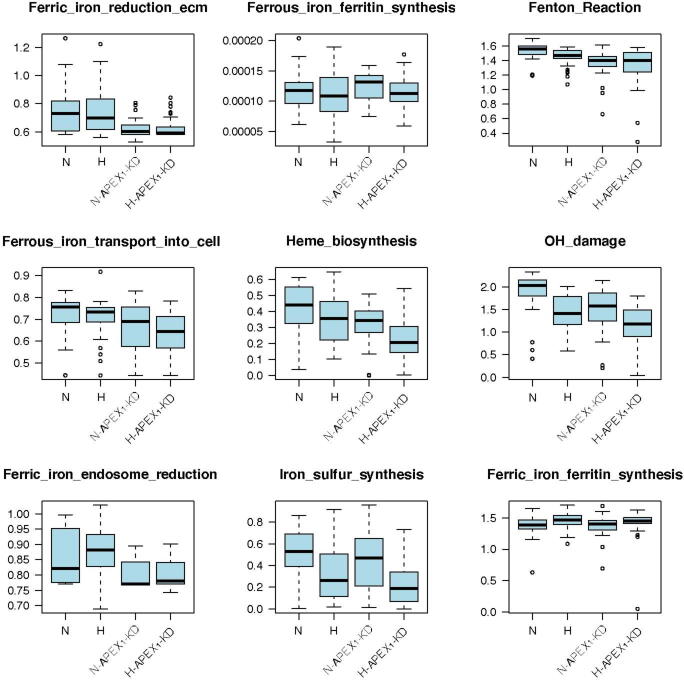
Acid–base homeostasis is a fundamental property of living cells, and its persistent disruption in human cells can lead to a wide range of diseases. In this study, we conducted a computational modeling analysis of transcriptomic data of 4750 human tissue samples of 9 cancer types in The Cancer Genome Atlas (TCGA) database. Built on our previous study, we quantitatively estimated the average production rate of OH− by cytosolic Fenton reactions, which continuously disrupt the intracellular pH (pHi) homeostasis. Our predictions indicate that all or at least a subset of 43 reprogrammed metabolisms (RMs) are induced to produce net protons (H+) at comparable rates of Fenton reactions to keep the pHi stable. We then discovered that a number of well-known phenotypes of cancers, including increased growth rate, metastasis rate, and local immune cell composition, can be naturally explained in terms of the Fenton reaction level and the induced RMs. This study strongly suggests the possibility to have a unified framework for studies of cancer-inducing stressors, adaptive metabolic reprogramming, and cancerous behaviors. In addition, strong evidence is provided to demonstrate that a popular view that Na+/H+ exchangers along with lactic acid exporters and carbonic anhydrases are responsible for the intracellular alkalization and extracellular acidification in cancer may not be justified.
酸碱平衡及其对癌症表型行为的影响
酸碱平衡是活细胞的基本特性,人类细胞中酸碱平衡的持续破坏会导致多种疾病。我们对癌症基因组图谱(TCGA)数据库中九种癌症类型的 4750 份人体组织样本的转录组数据进行了计算建模和分析。在之前研究的基础上,我们对持续破坏细胞内 pH 平衡的细胞膜芬顿反应产生 OH- 的(平均)速率进行了定量估算。我们的预测表明,43 种重编程代谢物(RMs)中的全部或一部分都会被诱导以相当的芬顿反应速率产生净质子(H+),以保持细胞内 pH 值的稳定。我们随后发现,一些众所周知的癌症表型,包括生长率、转移率和局部免疫细胞组成的增加,都可以用芬顿反应水平和诱导的 RMs 来自然地解释。这项研究有力地表明,有可能为癌症诱导应激源、适应性代谢重编程和癌症行为的研究建立一个统一的框架。此外,该研究还提供了有力的证据,证明了Na+/H+交换体与乳酸输出体和碳酸酐酶共同负责癌症细胞内碱化和细胞外酸化的普遍观点可能是不正确的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Genomics, Proteomics & Bioinformatics
Biochemistry, Genetics and Molecular Biology-Biochemistry
CiteScore
14.30
自引率
4.20%
发文量
844
审稿时长
61 days
期刊介绍:
Genomics, Proteomics and Bioinformatics (GPB) is the official journal of the Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China. It aims to disseminate new developments in the field of omics and bioinformatics, publish high-quality discoveries quickly, and promote open access and online publication. GPB welcomes submissions in all areas of life science, biology, and biomedicine, with a focus on large data acquisition, analysis, and curation. Manuscripts covering omics and related bioinformatics topics are particularly encouraged. GPB is indexed/abstracted by PubMed/MEDLINE, PubMed Central, Scopus, BIOSIS Previews, Chemical Abstracts, CSCD, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


