Eph receptor B2 (EPHB2) regulates cancer stem cell-like properties in hepatocellular carcinoma.
Q1 Biochemistry, Genetics and Molecular Biology
Stem cell investigation
Pub Date : 2022-10-19
eCollection Date: 2022-01-01
DOI:10.21037/sci-2022-031
引用次数: 0
Abstract
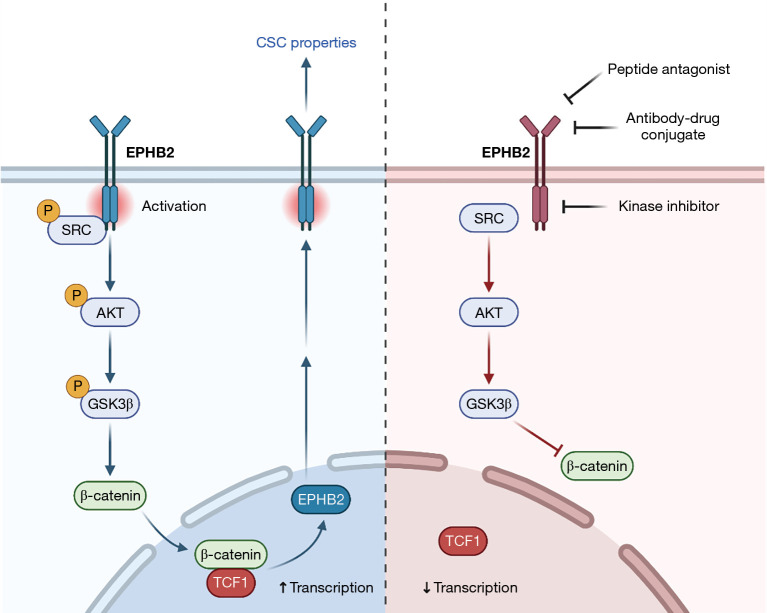
© Stem Cell Investigation. All rights reserved. Stem Cell Investig 2022;9:5 | https://dx.doi.org/10.21037/sci-2022-031 Hepatocellular carcinoma (HCC) is the most common primary liver cancer with high mortality rates globally. Poor prognosis is associated with eventual treatment resistance and disease relapse, which are often attributed to the presence of a small subset of tumor cells characterized by stem cell-like phenotypes, commonly known as cancer stem cells (CSCs). CSCs possess several key traits such as self-renewing potential, ability to initiate tumor growth, and resistance to conventional therapies targeted at rapidly proliferating cells that form the tumor bulk. In recent years, significant advances have been made in understanding the biology of different tumor CSCs and their corresponding markers, as well as how they interact with the tumor microenvironment. As increasing evidence show that long-term tumor eradication requires eliminating not just the proliferative cells but also the tumor-initiating subpopulations (1,2), greater focus has been placed on developing therapeutic strategies that target CSCs, with potential for future clinical implementation. An important aspect of specifically targeting these tumor-initiating cells rely on the use of cell surface markers. Studies on liver CSCs have uncovered various cell surface antigens such as EpCAM, CD90, CD47, CD13, CD24, and CD133 that identify subpopulations of tumor-initiating cells in HCC (3-8). However, not all cell surface markers are functionally involved in regulating CSC phenotypes in HCC, and specific small molecule inhibitors against them are scarce. In a recent study by Leung et al. (9), the authors identified a novel CSC target for HCC, Eph receptor B2 (EPHB2), that not only acts as a membrane-bound CSC marker but also plays regulatory roles in promoting stemness properties in liver tumor cells. EPHB2 expression was found to increase progressively from normal liver to cirrhotic liver and to HCC in human and mouse models (9). EPHB2 belongs to a large family of Eph receptor tyrosine kinases, which associate with membranebound ephrin ligands through cell-cell contact, leading to bidirectional intracellular signaling and activation of subsequent downstream signaling cascades (10). Eph receptors are involved in various cellular functions and processes, including cellular adhesion, cell migration, vascular development, cell proliferation and survival (10). The complexity of Eph receptor signaling also extends to its involvement in cancer. In fact, the role of EPHB2 in cancer is controversial, as it has been demonstrated to play both tumor-promoting and tumor-suppressive functions depending on the cellular context. In cancers such as glioblastoma, breast cancer, cervical cancer, and cutaneous squamous cell carcinoma, EPHB2 is overexpressed and promotes tumor progression and invasiveness (11-14). In contrast, EPHB2 exerts inhibitory effects on tumor cell migration and invasion, along with its downregulated expression, in cancers of the bladder and colon (15,16). In the context of CSCs, few studies have investigated EPHB2’s role in regulating cancer stemness. EPHB2 was found to be highly expressed in colorectal cancer cells with intestinal stem cell-like phenotypes, and its expression gradually decreased as the cells became differentiated (17,18). In cervical cancer, EPHB2 was involved in orchestrating an epithelial-mesenchymal transition (EMT) program which Editorial Commentary
Eph受体B2 (EPHB2)在肝细胞癌中调控肿瘤干细胞样特性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Stem cell investigation
Biochemistry, Genetics and Molecular Biology-Developmental Biology
CiteScore
5.80
自引率
0.00%
发文量
9
期刊介绍:
The Stem Cell Investigation (SCI; Stem Cell Investig; Online ISSN: 2313-0792) is a free access, peer-reviewed online journal covering basic, translational, and clinical research on all aspects of stem cells. It publishes original research articles and reviews on embryonic stem cells, induced pluripotent stem cells, adult tissue-specific stem/progenitor cells, cancer stem like cells, stem cell niche, stem cell technology, stem cell based drug discovery, and regenerative medicine. Stem Cell Investigation is indexed in PubMed/PMC since April, 2016.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


