Comparing immunogenicity and efficacy of two different mRNA-based COVID-19 vaccines as a fourth dose; six-month follow-up, Israel, 27 December 2021 to 24 July 2022.
IF 7.8
Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin
Pub Date : 2022-09-01
DOI:10.2807/1560-7917.ES.2022.27.39.2200701
引用次数: 4
Abstract
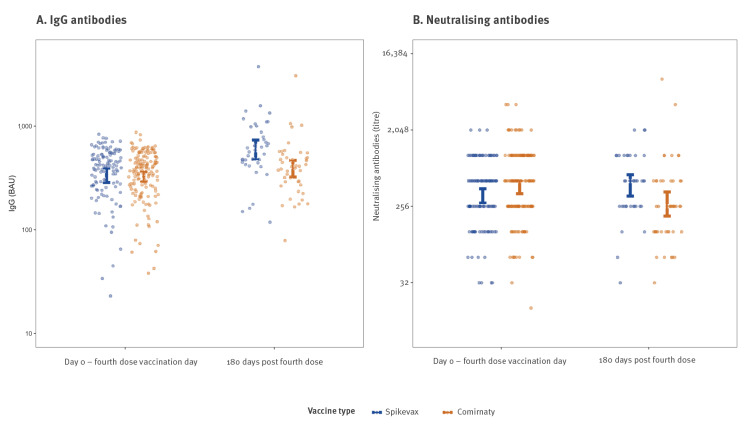
We assess the immunogenicity and efficacy of Spikevax and Comirnaty as fourth dose COVID-19 vaccines. Six months post-fourth-dose, IgG levels were higher than pre-fourth dose at 1.58-fold (95% CI: 1.27-1.97) in Spikevax and 1.16-fold (95% CI: 0.98-1.37) in Comirnaty vaccinees. Nearly 60% (159/274) of vaccinees contracted SARS-CoV-2. Infection hazard ratios (HRs) for Spikevax (0.82; 95% CI: 0.62-1.09) and Comirnaty (0.86; 95% CI: 0.65-1.13) vaccinees were similar, as were substantial-disease HRs, i.e. 0.28 (95% CI: 0.13-0.62) and 0.51 (95% CI: 0.27-0.96), respectively.
比较两种不同mRNA-based COVID-19疫苗作为第四剂的免疫原性和疗效六个月后续行动,以色列,2021年12月27日至2022年7月24日。
我们评估了Spikevax和Comirnaty作为第四剂COVID-19疫苗的免疫原性和有效性。第四次接种后6个月,Spikevax疫苗的IgG水平比第四次接种前高1.58倍(95% CI: 1.27-1.97), community疫苗的IgG水平比第四次接种前高1.16倍(95% CI: 0.98-1.37)。近60%(159/274)的疫苗接种者感染了SARS-CoV-2。Spikevax的感染风险比(hr)为0.82;95% CI: 0.62-1.09)和共伴性(0.86;95% CI: 0.65-1.13)疫苗接种者相似,实质疾病hr也相似,分别为0.28 (95% CI: 0.13-0.62)和0.51 (95% CI: 0.27-0.96)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


