Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes
IF 10.1
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 47
Abstract
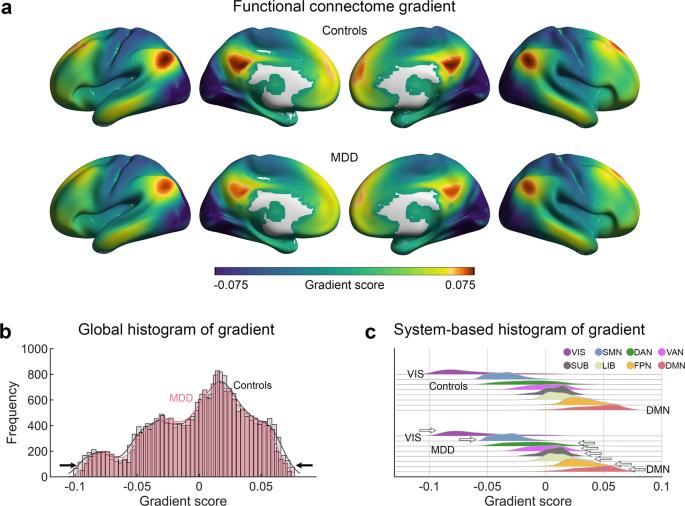
Patients with major depressive disorder (MDD) exhibit concurrent deficits in both sensory and higher-order cognitive processing. Connectome studies have suggested a principal primary-to-transmodal gradient in functional brain networks, supporting the spectrum from sensation to cognition. However, whether this gradient structure is disrupted in patients with MDD and how this disruption associates with gene expression profiles and treatment outcome remain unknown. Using a large cohort of resting-state fMRI data from 2227 participants (1148 MDD patients and 1079 healthy controls) recruited at nine sites, we investigated MDD-related alterations in the principal connectome gradient. We further used Neurosynth, postmortem gene expression, and an 8-week antidepressant treatment (20 MDD patients) data to assess the meta-analytic cognitive functions, transcriptional profiles, and treatment outcomes related to MDD gradient alterations, respectively. Relative to the controls, MDD patients exhibited global topographic alterations in the principal primary-to-transmodal gradient, including reduced explanation ratio, gradient range, and gradient variation (Cohen’s d = 0.16–0.21), and focal alterations mainly in the primary and transmodal systems (d = 0.18–0.25). These gradient alterations were significantly correlated with meta-analytic terms involving sensory processing and higher-order cognition. The transcriptional profiles explained 53.9% variance of the altered gradient pattern, with the most correlated genes enriched in transsynaptic signaling and calcium ion binding. The baseline gradient maps of patients significantly predicted symptomatic improvement after treatment. These results highlight the connectome gradient dysfunction in MDD and its linkage with gene expression profiles and clinical management, providing insight into the neurobiological underpinnings and potential biomarkers for treatment evaluation in this disorder.
重度抑郁症患者的连接组梯度功能障碍及其与基因表达谱和治疗效果的关系
重度抑郁症(MDD)患者同时表现出感觉和高阶认知处理能力的缺陷。连接组研究表明,大脑功能网络中存在一个从初级到中级的梯度,支持从感觉到认知的频谱。然而,这种梯度结构在 MDD 患者中是否受到破坏,以及这种破坏如何与基因表达谱和治疗结果相关联,这些仍是未知数。我们利用在九个地点招募的 2227 名参与者(1148 名 MDD 患者和 1079 名健康对照者)的大量静息态 fMRI 数据,研究了与 MDD 相关的主要连接组梯度的改变。我们还进一步利用 Neurosynth、死后基因表达和为期 8 周的抗抑郁治疗(20 名 MDD 患者)数据,分别评估了与 MDD 梯度改变相关的元分析认知功能、转录特征和治疗结果。与对照组相比,MDD 患者的主要初级-跨模态梯度表现出整体地形改变,包括解释比、梯度范围和梯度变化减少(Cohen's d = 0.16-0.21),病灶改变主要集中在初级和跨模态系统(d = 0.18-0.25)。这些梯度变化与涉及感觉处理和高阶认知的元分析术语明显相关。转录图谱解释了梯度模式改变的 53.9% 方差,其中相关性最强的基因集中在跨突触信号转导和钙离子结合。患者的基线梯度图可以显著预测治疗后症状的改善。这些结果突显了多发性硬化症患者的连接组梯度功能障碍及其与基因表达谱和临床管理的联系,为评估这种疾病的治疗提供了神经生物学基础和潜在生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


