Structural basis of ion permeation gating in Slo2.1 K+ channels.
引用次数: 25
Abstract
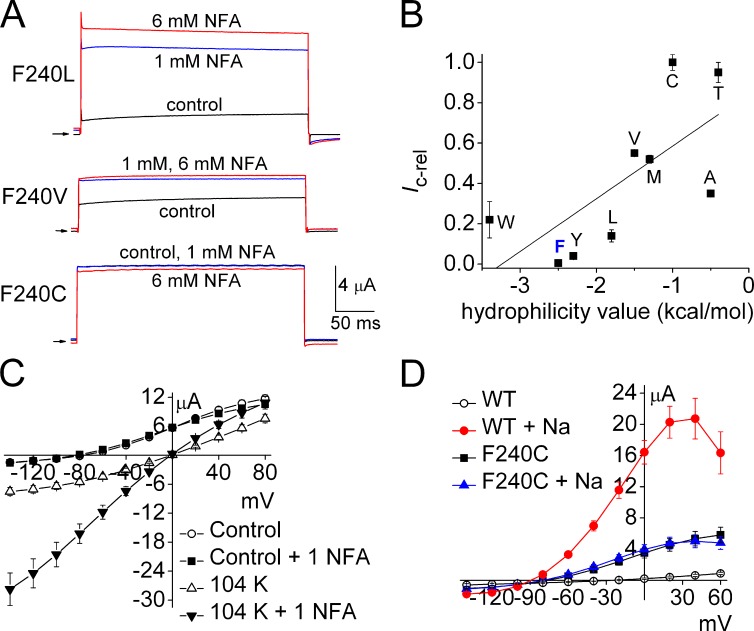
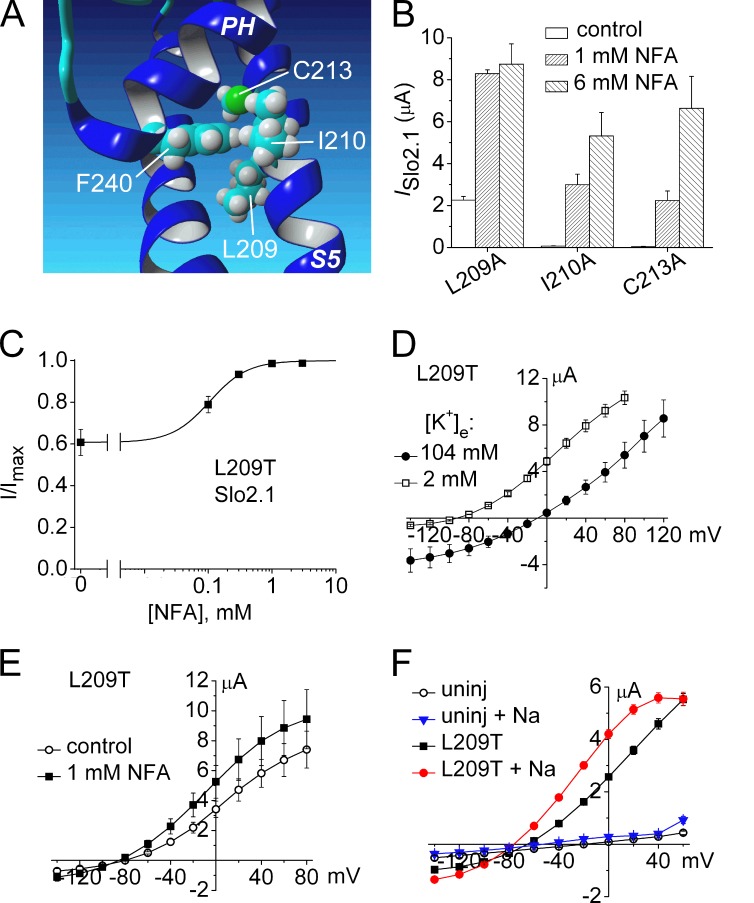
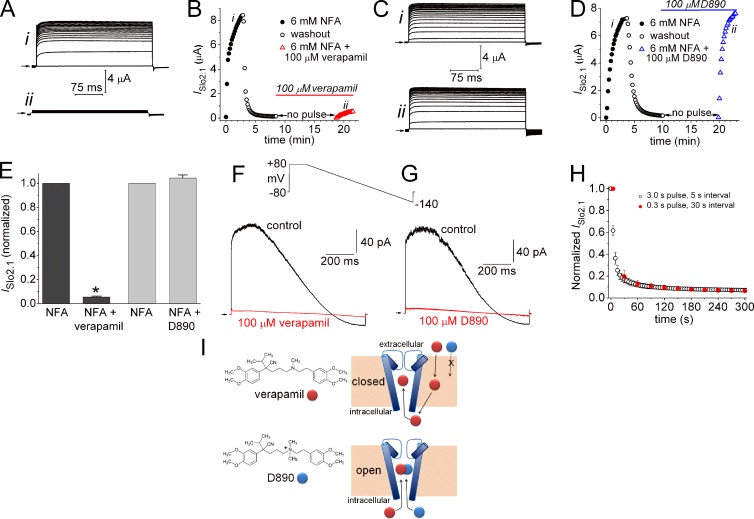
The activation gate of ion channels controls the transmembrane flux of permeant ions. In voltage-gated K+ channels, the aperture formed by the S6 bundle crossing can widen to open or narrow to close the ion permeation pathway, whereas the selectivity filter gates ion flux in cyclic-nucleotide gated (CNG) and Slo1 channels. Here we explore the structural basis of the activation gate for Slo2.1, a weakly voltage-dependent K+ channel that is activated by intracellular Na+ and Cl−. Slo2.1 channels were heterologously expressed in Xenopus laevis oocytes and activated by elevated [NaCl]i or extracellular application of niflumic acid. In contrast to other voltage-gated channels, Slo2.1 was blocked by verapamil in an activation-independent manner, implying that the S6 bundle crossing does not gate the access of verapamil to its central cavity binding site. The structural basis of Slo2.1 activation was probed by Ala scanning mutagenesis of the S6 segment and by mutation of selected residues in the pore helix and S5 segment. Mutation to Ala of three S6 residues caused reduced trafficking of channels to the cell surface and partial (K256A, I263A, Q273A) or complete loss (E275A) of channel function. P271A Slo2.1 channels trafficked normally, but were nonfunctional. Further mutagenesis and intragenic rescue by second site mutations suggest that Pro271 and Glu275 maintain the inner pore in an open configuration by preventing formation of a tight S6 bundle crossing. Mutation of several residues in S6 and S5 predicted by homology modeling to contact residues in the pore helix induced a gain of channel function. Substitution of the pore helix residue Phe240 with polar residues induced constitutive channel activation. Together these findings suggest that (1) the selectivity filter and not the bundle crossing gates ion permeation and (2) dynamic coupling between the pore helix and the S5 and S6 segments mediates Slo2.1 channel activation.
Slo2.1 K+通道离子渗透门控的结构基础。
离子通道的激活门控制渗透离子的跨膜通量。在电压门控K(+)通道中,S6束交叉形成的孔径可以变宽打开或变窄关闭离子渗透途径,而在环核苷酸门控(CNG)和Slo1通道中,选择性过滤器可以控制离子通量。在这里,我们探索了Slo2.1激活门的结构基础,Slo2.1是一个弱电压依赖性的K(+)通道,由细胞内Na(+)和Cl(-)激活。Slo2.1通道在非洲爪蟾卵母细胞中异种表达,并通过升高[NaCl]i或细胞外应用尼氟替酸激活。与其他电压门控通道相比,维拉帕米以激活无关的方式阻断Slo2.1,这意味着S6束的交叉不会阻止维拉帕米进入其中央腔结合位点。通过S6片段的Ala扫描诱变以及孔螺旋和S5片段中选定残基的突变,探索了Slo2.1激活的结构基础。三个S6残基的Ala突变导致通道向细胞表面运输减少,部分(K256A, I263A, Q273A)或完全(E275A)通道功能丧失。P271A Slo2.1通道传输正常,但功能不正常。进一步的诱变和第二位点突变的基因内挽救表明,Pro271和Glu275通过防止形成紧密的S6束交叉,使内孔保持开放状态。同源性模型预测S6和S5中几个残基与孔螺旋中的接触残基发生突变,导致通道函数增加。用极性残基取代孔螺旋残基Phe240诱导本构通道激活。综上所述,这些发现表明:(1)选择性过滤而非束交叉门离子渗透;(2)孔螺旋与S5和S6段之间的动态耦合介导了Slo2.1通道的激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


