Probabilistic modelling of developmental neurotoxicity based on a simplified adverse outcome pathway network
Abstract
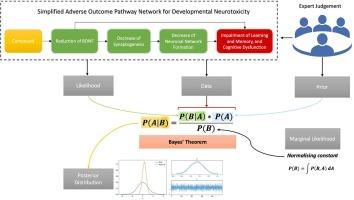
In a century where toxicology and chemical risk assessment are embracing alternative methods to animal testing, there is an opportunity to understand the causal factors of neurodevelopmental disorders such as learning and memory disabilities in children, as a foundation to predict adverse effects. New testing paradigms, along with the advances in probabilistic modelling, can help with the formulation of mechanistically-driven hypotheses on how exposure to environmental chemicals could potentially lead to developmental neurotoxicity (DNT). This investigation aimed to develop a Bayesian hierarchical model of a simplified AOP network for DNT. The model predicted the probability that a compound induces each of three selected common key events (CKEs) of the simplified AOP network and the adverse outcome (AO) of DNT, taking into account correlations and causal relations informed by the key event relationships (KERs). A dataset of 88 compounds representing pharmaceuticals, industrial chemicals and pesticides was compiled including physicochemical properties as well as in silico and in vitro information. The Bayesian model was able to predict DNT potential with an accuracy of 76%, classifying the compounds into low, medium or high probability classes. The modelling workflow achieved three further goals: it dealt with missing values; accommodated unbalanced and correlated data; and followed the structure of a directed acyclic graph (DAG) to simulate the simplified AOP network. Overall, the model demonstrated the utility of Bayesian hierarchical modelling for the development of quantitative AOP (qAOP) models and for informing the use of new approach methodologies (NAMs) in chemical risk assessment.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


