The importance of regulation (EU) 2017/746 for quality control in medical laboratories.
IF 1.8
3区 医学
Q1 MEDICAL LABORATORY TECHNOLOGY
引用次数: 1
Abstract
The guideline C24 (now in its 4th edition) issued by the Clinical and Laboratory Standards Institute (CLSI) recommends that for purchased quality control (QC), the laboratory should never use the manufacturer’s declared target (T) value but that identified by testing the product at least ten times with each new batch (1). Of course, verifying T is quite different from estimating the variability of the analytical process, albeit both are used for the statistical process control (SPC) since T should be the production target of the QC material on which the manufacturer has complete control.
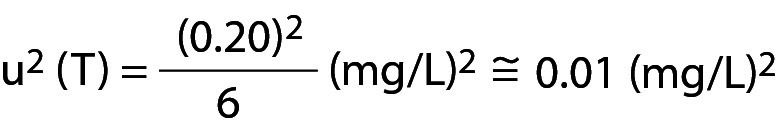
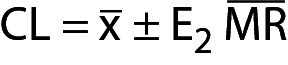
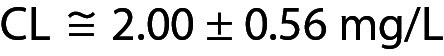
法规(EU) 2017/746对医学实验室质量控制的重要性
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biochemia Medica
医学-医学实验技术
CiteScore
5.50
自引率
3.00%
发文量
70
审稿时长
>12 weeks
期刊介绍:
Biochemia Medica is the official peer-reviewed journal of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Journal provides a wide coverage of research in all aspects of clinical chemistry and laboratory medicine. Following categories fit into the scope of the Journal: general clinical chemistry, haematology and haemostasis, molecular diagnostics and endocrinology. Development, validation and verification of analytical techniques and methods applicable to clinical chemistry and laboratory medicine are welcome as well as studies dealing with laboratory organization, automation and quality control. Journal publishes on a regular basis educative preanalytical case reports (Preanalytical mysteries), articles dealing with applied biostatistics (Lessons in biostatistics) and research integrity (Research integrity corner).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


