Misrouting of glucagon and stathmin-2 towards lysosomal system of α-cells in glucagon hypersecretion of diabetes.
IF 1.7
4区 医学
Q3 ENDOCRINOLOGY & METABOLISM
引用次数: 3
Abstract
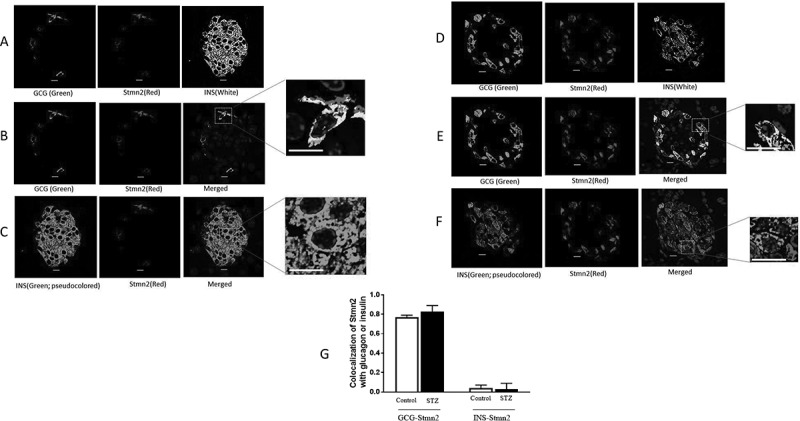
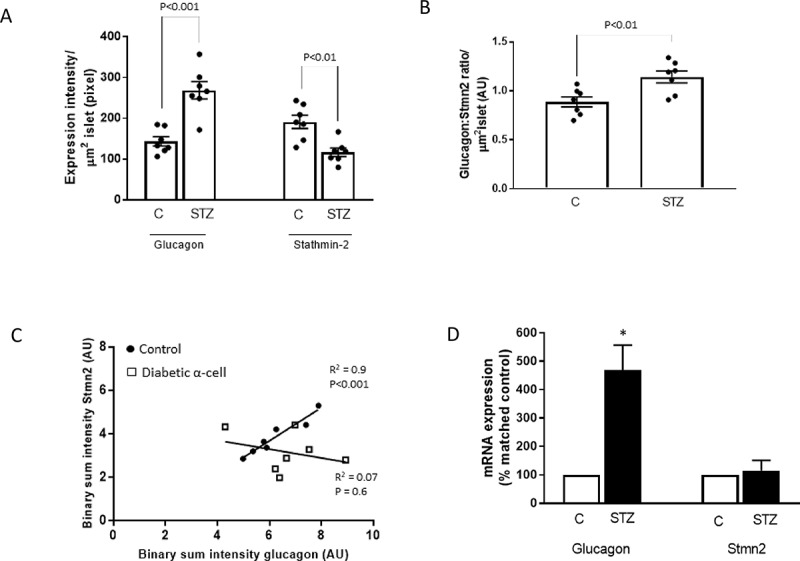
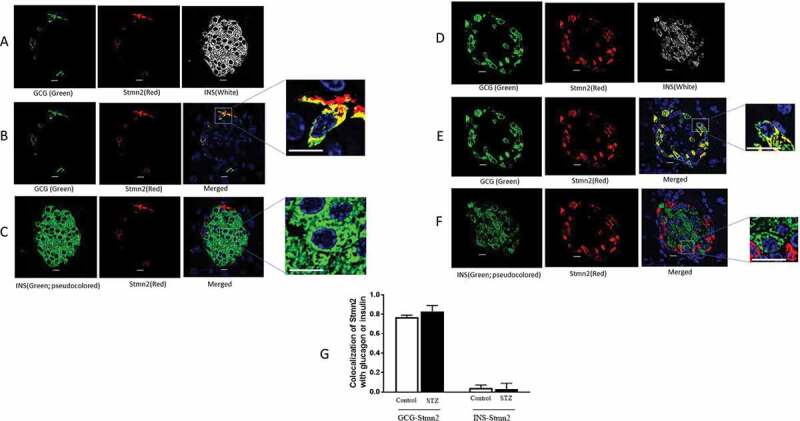
Glucagon hypersecretion from the pancreatic α-cell is a characteristic sign of diabetes, which exacerbates fasting hyperglycemia. Thus, targeting glucagon secretion from α-cells may be a promising approach for combating hyperglucagonemia. We have recently identified stathmin-2 as a protein that resides in α-cell secretory granules, and showed that it regulates glucagon secretion by directing glucagon towards the endolysosomal system in αTC1-6 cells. Here, we hypothesized that disruption of Stmn2-mediated trafficking of glucagon to the endolysosomes contributes to hyperglucagonemia. In isolated islets from male mice treated with streptozotocin (STZ) to induce diabetes, Arg-stimulated secretion of glucagon and Stmn2 was augmented. However, cell glucagon content was significantly increased (p<0.001), but Stmn2 levels were reduced (p<0.01) in STZ-treated mice, as measured by both ELISA and immunofluorescence intensity. Expression of Gcg mRNA increased ~4.5 times, while Stmn2 mRNA levels did not change. Using confocal immunofluorescence microscopy, the colocalization of glucagon and Stmn2 in Lamp2A+ lysosomes was dramatically reduced (p<0.001) in islets from diabetic mice, and the colocalization of Stmn2, but not glucagon, with the late endosome marker, Rab7, significantly (p<0.01) increased. Further studies were conducted in αTC1-6 cells cultured in media containing high glucose (16.7 mM) for two weeks to mimic glucagon hypersecretion of diabetes. Surprisingly, treatment of αTC1-6 cells with the lysosomal inhibitor bafilomycin A1 reduced K+-induced glucagon secretion, suggesting that high glucose may induce glucagon secretion from another lysosomal compartment. Both glucagon and Stmn2 co-localized with Lamp1, which marks secretory lysosomes, in cells cultured in high glucose. We propose that, in addition to enhanced trafficking and secretion through the regulated secretory pathway, the hyperglucagonemia of diabetes may also be due to re-routing of glucagon from the degradative Lamp2A+ lysosome towards the secretory Lamp1+lysosome.
糖尿病胰高血糖素高分泌中胰高血糖素和凝血素-2向α-细胞溶酶体系统的错误路径。
胰高血糖素分泌过多是糖尿病的特征性表现,它加剧了空腹高血糖。因此,靶向α-细胞分泌胰高血糖素可能是对抗高胰高血糖素血症的一种有希望的方法。我们最近发现stathin -2是一种α-细胞蛋白,通过将胰高血糖素导向αTC1-6细胞的内溶酶体系统来调节胰高血糖素的分泌。我们假设stmn2介导的胰高血糖素转运到糖尿病内溶酶体的破坏有助于高胰高血糖素血症。在雄性小鼠离体胰岛中,经链脲佐菌素(STZ)处理后,胰高血糖素分泌和细胞含量增加,但细胞Stmn2水平降低(p +溶酶体显著减少),提示高糖可能诱导另一溶酶体室分泌胰高血糖素。在高糖培养的细胞中,胰高血糖素和Stmn2与标记分泌溶酶体的Lamp1共定位。我们认为,糖尿病的高胰高血糖素血症除了通过受调节的分泌途径增强转运和分泌外,还可能是由于胰高血糖素从降解性的Lamp2A+溶酶体向分泌性的Lamp1+溶酶体重新转运。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Islets
ENDOCRINOLOGY & METABOLISM-
CiteScore
3.30
自引率
4.50%
发文量
10
审稿时长
>12 weeks
期刊介绍:
Islets is the first international, peer-reviewed research journal dedicated to islet biology. Islets publishes high-quality clinical and experimental research into the physiology and pathology of the islets of Langerhans. In addition to original research manuscripts, Islets is the leading source for cutting-edge Perspectives, Reviews and Commentaries.
Our goal is to foster communication and a rapid exchange of information through timely publication of important results using print as well as electronic formats.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


